This anonymous essay was submitted to Open Philanthropy's Cause Exploration Prizes contest and posted to the Forum with the authors' permission.
If you're seeing this in summer 2022, we'll be posting many submissions in a short period. If you want to stop seeing them so often, apply a filter for the appropriate tag!
Executive Summary
Sickle cell disease causes a significant amount of infant mortality, largely focused in specific countries in Sub-Saharan Africa. The vast majority of this mortality is preventable with early diagnosis, inexpensive prophylaxis and treatment. New diagnostic tests are now available which can be performed at the point of care and give results in minutes, removing a major barrier to establishing national newborn screening programmes. These new tests are also cheaper and easier to use than traditional methods, which means they can significantly improve the cost-effectiveness of addressing sickle cell disease.
The case for sickle cell disease as a cause area is strongest in terms of tractability and neglectedness. No major global health donor focuses on sickle cell disease – and this is a disease where we can easily make significant reductions in infant mortality with basic medicines and prophylaxis.
The case for sickle cell disease as a cause area is perhaps weaker in terms of importance, because it is challenging to disentangle the impact of sickle cell disease from other conditions it interacts with. The overall scale of the problem whilst large, is not enormous when set against extinction level risks or more widespread health challenges such as air pollution.
The problem is geographically specific, with certain countries in Sub-Saharan Africa, and certain ethnic groups in India facing the vast majority of disease burden. The amount of funding that this issue could absorb is not enormous, but it represents a highly cost-effective problem to tackle which could yield substantial results within a relatively short timeframe (less than 5 years).
Major Sources of Uncertainty
- Expanded testing for sickle cell disease may find more or less cases in the populations of Sub-Saharan Africa, therefore improving or decreasing the cost-effectiveness of interventions.
- Longitudinal cohort studies could give better estimates of mortality from sickle cell disease in Sub-Saharan Africa and links with other diseases such as malaria, pneumococcal sepsis and others.
- Evidence generation is needed to devise optimal testing algorithm using POC diagnostics (e.g. at what age can they distinguish fetal hemoglobin, is there a need for confirmatory test, how should these tests be integrated into existing health touchpoints like vaccination, what role should testing pre-conception play).
- Need to validate diagnostic tests in country setting and get these products approved by regulatory authorities in all contexts.
- Implementing a sustainable national newborn screening programme in Sub-Saharan African countries is a significant technical and logistical challenge, and will likely require significant technical assistance and engagement with country governments – this is not necessarily just a case of providing more money.
- Whilst reducing infant mortality is likely to be highly cost-effective, children who go on to live with sickle cell disease will require more medical treatments throughout their lives, particularly for pain management. The costs of this additional treatment are challenging to effectively model.
- Timeline for a potential cure to sickle cell disease. If a cure is available and reasonably affordable, then the later-in life costs of additional treatment after initial lifesaving support would be significantly reduced.
What is the problem?
Sickle cell disease (SCD) is the most common genetic disease in the world. Sufferers of the condition have abnormal hemoglobin in red blood cells (RBCs), causing them to ‘sickle’. This creates several severe and chronic complications which can be life threatening. The main symptoms of sickle cell disease are anemia, and episodes of severe pain. The primary causes of mortality in children are malaria, severe anemia and bacterial infections.
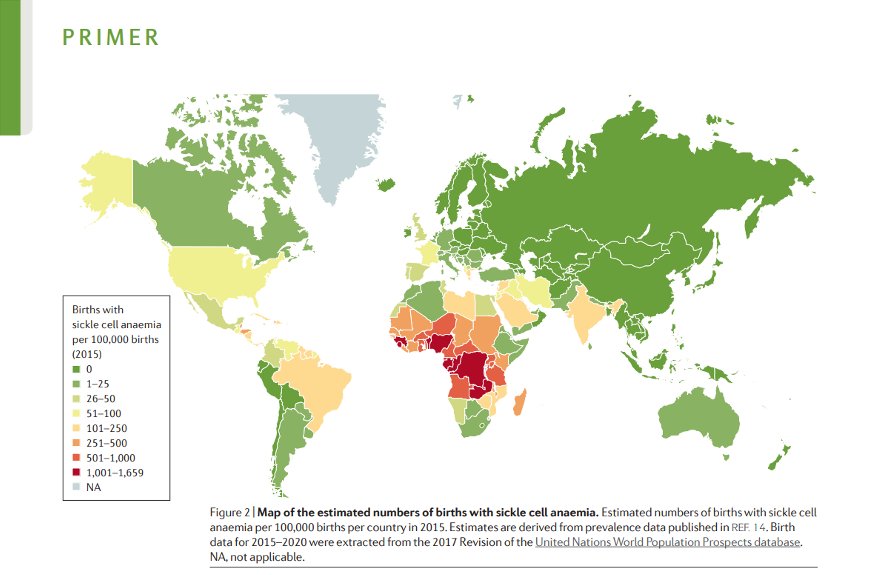
[1]
Current data is not conclusive, but SCD is estimated to result in a 50-90% mortality rate of children under 5 in Africa.[2] Approximately 300,000 children were born with sickle cell disease in 2010, and this is expected to increase to around 400,000 children by 2050 due to shifting demographics, predominantly in Sub-Saharan Africa[3].
Lifesaving treatment for sickle cell disease is cheap and cost-effective but no country in Africa has a successful program for comprehensive SCD care or national newborn screening. For instance, children with sickle cell anemia should be started on prophylactic penicillin as this reduces mortality from streptococcus pneumoniae by 84%[4], one of the major killers of children suffering from SCD.
The key interventions to reduce sickle cell disease mortality are early diagnosis[5], prophylaxis and close management. When babies are born in the USA or other wealthy countries, they can be screened as newborns for sickle cell disease using samples which are sent to advanced laboratories which can perform diagnostic tests. The equipment to perform High Performance Liquid Chromatography (HPLC) or isoelectric focusing (IEF) to diagnose sickle cell disease is expensive and is not widely available in Sub-Saharan Africa.
However recent advances in point of care, rapid diagnostic tests mean that newborns and infants can be screened for sickle cell disease without the need for a centralized laboratory, meaning that we can realistically envisage national newborn screening even in low-income settings. At least three point of care testing solutions currently exist and have evidence that they could support newborn screening: HemeChip Gazelle[6], Sickle Scan[7] & Hemotype SC[8].
Neglectedness
In 2006, the WHO said there was an urgent need to respond to sickle cell disease. Since then, perhaps 4 million babies have been born with the disease and probably most have died. Sickle cell disease is frequently described in the literature as “an invisible global health issue”[9] or a “silent epidemic”[10].
As far as I can tell, sickle cell disease is not a priority for any major health donor. Ironically but tragically, sickle cell disease is too neglected to even be classified as a neglected tropical disease (NTD) by donors and global health agencies[11]. The Pierre Fabre Foundation made an approximate estimate that just $20m was spent on sickle cell disease in 2018[12], which surely shows there is significant room to expand funding in this area.
Speculatively this could change in future, in particular due to the links between sickle cell disease and malaria. Malaria as a target disease of the Global Fund is significantly better funded than sickle cell disease, and patients with sickle cell disease are particularly vulnerable to malaria[13]. Philanthropic Research and action to address sickle cell disease would reduce malaria mortality and could potentially unlock resources from better funded donors.
What is currently being done?
The Bill and Melinda Gates Foundation (BMGF) has funded a relatively small-scale program for newborn testing in Ghana & is also exploring experimental gene therapy treatments with the National Institute of Health[14]. Surprisingly perhaps, the approach BMGF has taken is to focus on seeking a cure to the disease through scientific research, whilst acknowledging that the current burden of mortality is largely preventable through existing technologies and approaches.
The Pierre Fabre foundation has funded a small pilot program for sickle cell disease in the Democratic Republic of Congo, however this is only supporting three health zones, in a country of over 500 health zones[15].
No Sub-Saharan African country has established a national newborn screening program despite the clear success of similar initiatives in reducing infant mortality in developed countries and even middle-income countries with high burden of disease such as Jamaica[16]. Ghana probably has the most advanced screening program in Sub-Saharan Africa, however, is still only screening a small fraction of annual newborns (less than 5% in 2015)[17].
Importance
Calculating the impact of sickle cell disease burden globally is not easy. A back of the envelope calculation would suggest that if there are 300,000 children born each year with sickle cell disease, and 75% of them are in Sub-Saharan Africa[18], and 50-90% of those die before the age of 5 years, then the annual death toll from sickle cell disease for infants ranges between 112,500 and 202,500 per year for Sub-Saharan Africa alone.
A crucial caveat here is that sickle cell disease increases the mortality rate from other causes (bacterial infections, malaria, severe anemia) so it may not be fair to ascribe all these deaths to sickle cell disease alone.
Nevertheless, this number is significant. For comparison purposes, malaria was estimated to kill 627,000 people per year in 2020[19]. (It should be noted that there will be overlap between these two categories. Children with one sickle cell mutation have a protection against malaria, however children with both mutated alleles (i.e. with sickle cell disease) are more vulnerable to severe malaria.)
Tractability
There is widespread agreement amongst experts that infant mortality from sickle cell disease can be dramatically reduced through basic public health interventions, when the disease is diagnosed early. These interventions include screening of newborns, penicillin and folic acid as prophylaxis, pneumococcal immunization and education of parents/caregivers to identify spleen sequestration and advice to seek prompt medical attention in case of fever[20]. Relatively low-cost treatment options are also available, such as hydroxurea which costs around $300 per patient per year[21].
Experts estimate that even in low/middle income settings, the excess mortality from sickle cell disease could be reduced from 90% to just 5% through universal screening programmes, access to prophylaxis and treatment[22]. One study estimates large scale universal screening programmes could save approximately 9.8 million lives by 2050[23], the majority of which will be in sub-Saharan Africa[24].
The Jamaica Cohort study is a strong body of evidence of the impact that a coordinated national screening intervention can have outside of high income countries, albeit in a wealthier setting than Sub-Saharan Africa. The study found that overall mortality in children under 10 years old decreased from 17.6% to 1.8%[25].
As stated earlier, there are no national newborn screening programmes in Sub-Saharan Africa for sickle cell disease. Whilst prophylaxis is available in health clinics – without early diagnosis it will not be prescribed until too late. Treatments such as hydroxyurea are also not available because of lack of funding, and countries are reliant on donation programs such as the one established by Novartis in Ghana in 2019.
The Pierre Fabre foundation is one of the largest charities focused on sickle cell disease work in the world, but the scale at which it operates is clearly insufficient to the challenge of the disease. The organization claims to have performed 29,084 screenings in 2020[26] – but the number of babies born in Ghana alone is over 800,000 each year.
Cost-effectiveness
From my review, the cost-effectiveness literature is largely focused on the question of whether universal vs. targeted screening is appropriate in Western countries with relatively low levels of sickle cell disease. One study suggested a cost per life saved of screening and treatment for black infants in the United States of $3100/life saved[27], which is actually cheaper than Givewell’s estimate of malaria cost-effectiveness of $4500/life saved[28].
A detailed 2016 research study looked at the cost-effectiveness of newborn screening and prophylactic interventions for sickle cell disease and estimated that interventions would be highly cost-effective in 24 countries in Sub-Saharan Africa (average cost per DALY averted: US$184)[29]. In particular, Nigeria (cost per DALY: $157) and DR Congo (cost per DALY: $150) had highly cost-effective results, which is encouraging given they represent a large proportion of the disease burden. The study estimated that newborn screening is cost-effective as long as incidence exceeds 0.2% - 0.3%.
For various reasons, I think an updated study would show an improve cost-effectiveness on this baseline. This study was based on the input assumption that the cost of screening (using a technique called isoelectric focusing) of $9.90[30]/test based on evidence from Uganda. Isoelectric focusing requires taking bloodspot samples from newborns and then posting them to a laboratory with the necessary specialized equipment to run a test.
The study estimated the cost of universal screening to be $312m/year, with prophylaxis and treatment costs contributing another $202m/year, so the majority of costs come from the screening program.
However new point of care diagnostics are available for as little as $2 currently[31], and do not require sample transportation, as well as being simpler to implement. In addition, this price should be expected to reduce significantly if mass newborn screening were to take place as countries are able to negotiate a lower price per test in exchange for bulk orders.
As a comparison point – tests for HIV have dramatically reduced in price due to negotiated access deals backed by volume guarantees, for instance a recent deal negotiated by MedAccess, Clinton Health Access Initiative (CHAI) and a Chinese diagnostic company secured HIV self-tests for less than $1 (ex-works price)[32], a 50% reduction when compared to the most commonly used test[33].
The cost of newborn screening at current prices using these rapid diagnostic tests can be conservatively estimated as 30% less than the previous estimate for isoelectric focusing[34], meaning that the cost-effectiveness of the program should be significantly better than previously estimated. Further price reductions on rapid point of care tests are highly likely when procured at scale, and since diagnostic costs are the largest cost driver for the program – this means that the overall cost-effectiveness in the previous detailed academic study is likely to be significantly improved.
It should be noted that children who survive into adulthood with sickle cell disease are likely to utilize healthcare more frequently than those without the condition. Even in the developed world, there is a substantial life expectancy gap for those with sickle cell disease. Cost-effectiveness analysis struggles to account for these significant later in life healthcare costs. More expensive treatments for sickle cell disease such as blood transfusions, and more expensive treatment drugs such as crizanlizumab and voxelotor are likely to be unaffordable in low-income contexts at least until patents expire and generics are available.
What could a new philanthropist do?
A new philanthropist focusing on sickle cell disease should seek to establish newborn screening programs and expand access to treatment in targeted Sub-Saharan African countries in partnership with ministries of health where appropriate. The philanthropist should seek to build on whatever existing diagnostic infrastructure exists, but also prioritize point of care testing which has been transformative for other diseases such as HIV in terms of improving patient care in resource-limited settings, particularly in rural areas[35].
The countries with the highest numbers of children with sickle cell disease in Sub-Saharan Africa are:
- Nigeria
- Democratic Republic of Congo
- Angola
- Cameroon
- Uganda
- Tanzania
- Ghana
Note that India also has a significant population with SCD however is likely to require a different approach[36]. India also experiences a different variant of SCD, (HbE) – which not all POC technologies are able to diagnose accurately.
Starting in countries with the highest rates of SCD per child could be a good approach. The exact approach will need to be tailored to the country context. Any national scale newborn screening and treatment project would need to be coordinated with existing health actors within a country’s health system. The relative levels of health system capacity within Sub-Saharan Africa vary substantially.
In a country like Ghana, there is strong uptake of public health interventions such as vaccination and an existing national health insurance program with reasonable levels of coverage. Funding for sickle cell disease testing and treatment could subsidize these interventions and enable the government to offer them for free or at an affordable price to families. Development of clinical guidelines, training programs for clinicians on sickle cell treatment may be necessary, as well as training and guidance on rolling out new diagnostic methods.

In another country such as Democratic Republic of Congo, the government significantly underinvests in health and probably lacks the capacity to implement a national newborn screening. Working through Non-Governmental Organization (NGO) partners to deliver the program may be a more effective option until government capacity has increased.

In the long-run, the goal should be to add these cost-effective interventions into the existing health system, and shift away from reliance on donor funding.
In addition to this country support work, a new philanthropic donor should recognise that it is a significant actor, with significant market power in this neglected space. Negotiating price discounts on sickle cell disease diagnostic tests and treatment solutions such as hydroxyurea could be undertaken on a cross-country basis, backed by volume guarantees which could further improve the cost-effectiveness of these programmes and help make costs affordable to country governments to take on in the longer-term.
As the number of children surviving sickle cell disease increases, the market size for more advanced solutions to combat the disease will also increase. This will encourage private sector investment in this neglected disease area. Prospectively these new patients may be able to access generic versions of current expensive sickle cell treatments (crizanlizumab and voxelotor) or even potential gene-therapy treatments if these are developed and scale successfully.
A more selfish reason for acting
The above, hopefully, is a well-considered, compelling argument for prioritizing sickle cell disease.
There is also a selfish, or less altruistic, or more meta perspective for considering sickle cell disease for the effective altruism community. If you don’t feel that’s appropriate – maybe stop reading now and consider this essay on its other merits.
The effective altruism movement may consider itself antiracist or viewing all lives as equally valuable. But this is not necessarily how it is perceived. The ‘movement’ after all is predominantly one of white men who attended elite universities and live in the United States and the United Kingdom.
You may think that perception is unfair and inaccurate, but it exists[37]. Investing in sickle cell disease, one which predominantly affects people of colour, particularly those from regions of Africa, could be an effective way of changing that perception or demonstrating that it is unfair. Certainly, it is true that sickle cell disease has been neglected because of a lack of focus on diseases which predominantly affect non-European, non-American populations.
Acknowledgements
With thanks to Jassi Pannu for providing comments on a draft. The author of this essay wishes to remain anonymous, but has worked in global health for over 10 years and works for an organization interested in this topic.
References
[1] http://dx.doi.org/10.1038/nrdp.2018.10
[2] Sickle cell disease in Africa: a neglected cause of early childhood mortality - PubMed (nih.gov)
[3] Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN (2013) Global Burden of Sickle Cell Anaemia in Children under Five, 2010–2050: Modelling Based on Demographics, Excess Mortality, and Interventions. PLoS Med 10(7): e1001484. doi:10.1371/journal.pmed.1001484
[4] https://pubmed.ncbi.nlm.nih.gov/3086721/
[5] http://dx.doi.org/10.1038/nrdp.2018.10
[6] Multispectral Imaging for MicroChip Electrophoresis Enables Point-of-Care Newborn Hemoglobin Variant Screening (medrxiv.org)
[7] https://www.thelancet.com/action/showPdf?pii=S2352-3026%2820%2930143-5
[8] Implementing newborn screening for sickle cell disease as part of immunisation programmes in Nigeria: a feasibility study (thelancet.com)
[9] Sickle cell disease: Reducing the global disease burden - PubMed (nih.gov)
[10] Sickle Cell Disease Child Mortality - A Silent Epidemic in Nigeria: Issues in Political Economy | UNICEF Global Development Commons
[11] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3671937/
[12] FPF-annualreport-2019UK.pdf (fondationpierrefabre.org)
[13] https://pubmed.ncbi.nlm.nih.gov/20530796/
[14] https://www.gatesfoundation.org/ideas/articles/gene-therapy-mike-mccune
[15] https://www.fondationpierrefabre.org/en/countries/the-drc-introduces-a-national-plan-to-combat-sickle-cell-disease/
[16] Newborn Screening for Sickle Cell Disease in the Caribbean: An Update of the Present Situation and of the Disease Prevalence - PMC (nih.gov)
[17] http://dx.doi.org/10.1038/nrdp.2018.10
[18] https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(19)30364-X/fulltext
[19] https://www.who.int/news-room/fact-sheets/detail/malaria
[20] Sickle cell disease: Reducing the global disease burden - PubMed (nih.gov)
[21] https://qz.com/africa/1756292/norvatis-sickle-cell-disease-drug-too-expensive-for-africans/#:~:text=African%20reality&text=In%20comparison%20Hydroxyurea%20is%20taken,the%20drug%20at%20around%20%24300.
[22] Global Burden of Sickle Cell Anaemia in Children under Five, 2010–2050: Modelling Based on Demographics, Excess Mortality, and Interventions - PMC (nih.gov)
[23] Global Burden of Sickle Cell Anaemia in Children under Five, 2010–2050: Modelling Based on Demographics, Excess Mortality, and Interventions - PMC (nih.gov)
[24] The paper’s estimate is for a 40 year period, so ~250,000 lives saved per year.
[25] Newborn Screening for Sickle Cell Disease in Jamaica: A Review – Past, Present and Future - PMC (nih.gov)
[26] Combating Sickle cell disease | Fondation Pierre Fabre
[27] Neonatal screening for sickle cell disease: a cost-effectiveness analysis - PubMed (nih.gov)
[28] Why Is It So Expensive to Save Lives? | GiveWell
[29] Kuznik et al. BMC Health Services Research (2016) 16:304 DOI 10.1186/s12913-016-1572-6
[30] In 2014 US dollars
[31] Inexpensive, rapid test might transform sickle cell screening | NHLBI, NIH
[32] Price of HIV self-tests lowered to $1 | Devex
[33] Four takeaways from the AIDS 2022 Conference - Clinton Health Access Initiative
[34] Prices of reagents, sample collection costs, instrument costs & sample transport were estimated at ~$5 in the study. These costs could be replaced by a single rapid test for under $2 (depending on need for confirmatory positive test). The study also estimated an additional $5 for staff time, overheads and facility rental – which could also be reduced with these simpler to operate tests which require fewer overheads and can be conducted in existing health facilities, without the need for advanced laboratories.
[35] Access and Quality of HIV-Related Point-of-Care Diagnostic Testing in Global Health Programs - PubMed (nih.gov)
[36] As the map above shows, the rate of sickle cell disease in India is lower than in high-burden countries in Sub-Saharan Africa, however due to the population size of India, there are a significant number of cases. These cases are concentrated in certain ethnic groups, and so the optimal strategy in India may be quite different to African countries.
[37] Effective Altruism Is The Nerdy Social Movement That Teaches People How To Do Good Better (forbes.com)
I spent a weekend at Google talking with nerds about charity. I came away … worried. - Vox

We are trying to raise funds for families living with children with this condition in Cameroon. We have written about our position in this post Critique our position – Sickle cell anaemia in sub-Saharan Africa (Cameroon) — EA Forum (effectivealtruism.org)
We hope you don't mind if we include our donation link, in case anyone wants to support these children: Help children with sickle cell syndrome - GlobalGiving
We realize your post is about sickle cell as a cause from a Public Health point of view while our approach is more of a Social Protection one.
There is substantial evidence that the prevalence of Sickle Cell characteristics in SubSaharan Africa is a direct and evolutionary response to Malaria's prevalence across the continent. https://www.pnas.org/doi/10.1073/pnas.1804388115
Those with Sickle Cell traits have been able to avoid death from malaria due to the reduced oxygenation of red blood cells due to this genetic mutation.
While intervention in Sickle Cell Anemic patients is important, attempts to "cure" sickle cell characteristics could have the unexpected consequence of increasing deaths from Malaria by "curing" this evolutionary benefit of this characteristic in Malarian areas.
As I understand it, you want to screen out people with two copies of the allele (i.e. who are at risk for sickle cell disease) but not people who have one copy (i.e. people who aren't at risk but still have resistance to malaria). So this doesn't really seem like a problem to me? I.e. if you screen 100% of people, you can eradicate sickle cell disease, but people will still have and pass on the allele that gives malaria resistance.