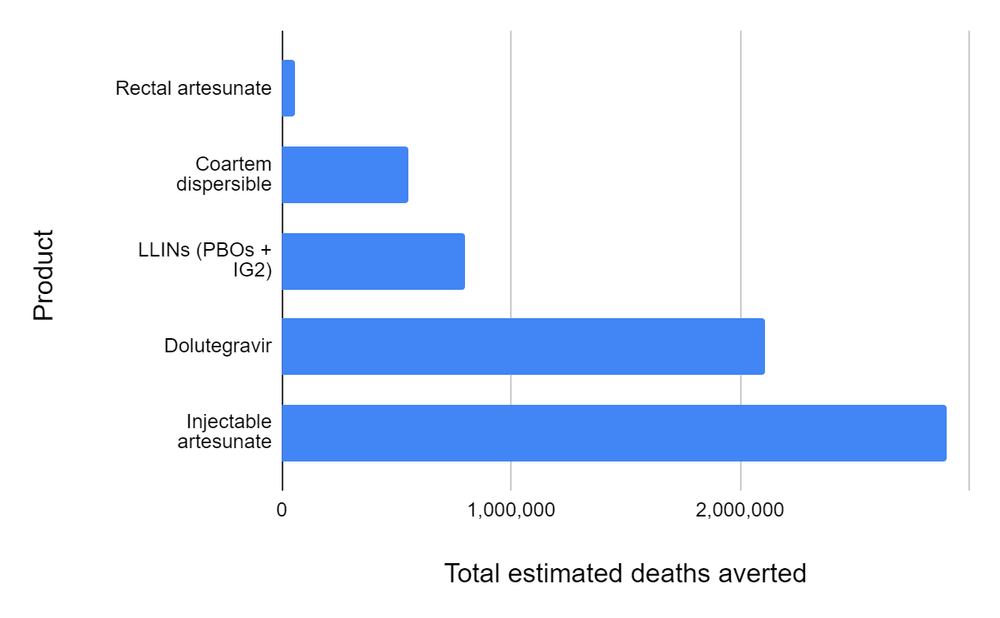Editorial note
This report was produced by Rethink Priorities during December 2022 and January 2023. The project was commissioned and supported by Open Philanthropy, which does not necessarily endorse our conclusions.
The report focuses on the development story and impact of some global health R&D ‘hits’ or ‘success stories’ of the last two decades: injectable artesunate, rectal artesunate, Coartem Dispersible, long-lasting insecticidal nets, and dolutegravir. We did not classify these developments as ‘hits’ ourselves, or compare them to any other treatments, but we were asked to look into them based on the expectation that they have saved or could save many lives, and could constitute some of the most successful treatments of the past couple of decades. We analyzed the steps that led to their development, with a particular focus on the roles different stakeholders played. Additionally, we estimated the number of deaths these products have averted to date and might avert in the future.
We have tried to flag major sources of uncertainty in the report and are open to revising our views as more information becomes available.
Executive summary
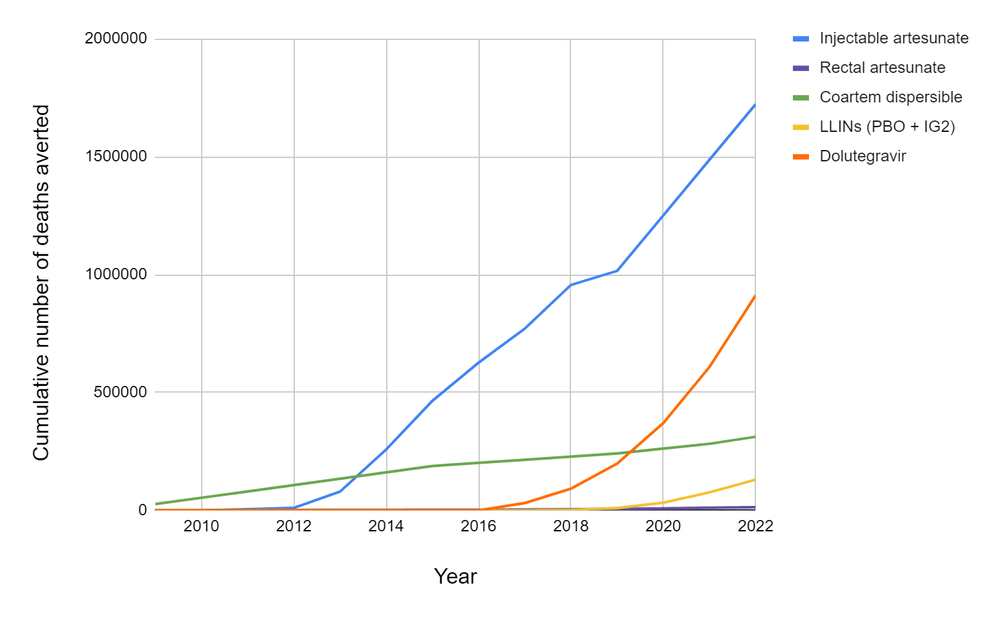
- In this report, we attempted to reconstruct the development story behind five global health R&D hits of the last two decades, four types of antimalarial treatments and one antiretroviral used for HIV, and estimate the number of deaths each of them has averted to date and might avert in total. The latter ranges from a few tens of thousands (for rectal artesunate) to a few million (for injectable artesunate and dolutegravir). You can find below two plots (Figures 1 and 2) summarizing our models’ estimates for the lives saved by each of these products since their introduction and until obsolescence (you can also find the plots and associated data here).
Figure 1: Cumulative number of deaths averted by each product since its introduction
Figure 2: Total number of deaths averted by each product until obsolescence
- In most of the examples studied, the drugs were developed through partnerships involving: (1) a pharmaceutical company that carried out the development of the product, (2) academics who ran the trials to show the product’s effects and/or compare it to the standard of care, and (3) philanthropic and public actors that provided funding for various stages. Philanthropic funds were also used in some cases to support local manufacturers and help them get WHO approval. One notable exception is dolutegravir, for which the development seems to have been entirely funded by industry.
- You can find here a table including the partners involved in the development of each of the products, and the cost and funders for different parts of the process where we were able to find the information. As noted above, philanthropic funds seem to have been pivotal to the development of all products except for dolutegravir. Even though the cost incurred by the pharmaceutical companies is unknown, and likely to be quite large, it seems as though these partnerships are quite frequent and necessary when developing products for diseases mainly affecting low and middle income countries.
- For all cases in which philanthropic funding played a significant role, the funds needed seem to have been in the range of a few million dollars to the low tens of millions. It should be noted that the total funds these organizations invested toward R&D of products related to these diseases is likely much larger, including funds devoted to these particular drugs through unsuccessful initiatives we were not able to find, and funds devoted to the development of other drugs that were unsuccessful (or just less successful).
Click here for the full version of this report on the Rethink Priorities website.
Contributions and acknowledgments
Bruce Tsai and Erin Braid were the main authors of this report. Erin Braid and Melanie Basnak edited the client-facing version of the report to transform it into a public-facing report. Melanie Basnak reviewed and supervised this report. Thanks to Adam Papineau for copyediting and to Rachel Norman for assistance with publishing the report online. Further thanks to Christopher Larkin, Mathias Mondy, and Tom McLean from IVCC for taking the time to speak with us.