“Clean meat”, or meat grown independently from an animal nervous system, is one of the most promising interventions for reducing animal suffering; if we can more cheaply create meat without hurting animals, then market forces could reduce or eliminate factory farming for good. In this talk from EA Global 2018: San Francisco, Marie Gibbons describes the technical details of scientific progress toward clean meat, and Natalie Cargill explores recent surveys of public opinion on the topic.
A transcript of their talk is below, including questions from the audience, which we have lightly edited for clarity. You can also watch it on YouTube and read it on effectivealtruism.org.
Marie's Talk
I'm going to be talking to you guys a little bit about the technical challenges that are associated with scaling up clean meat.
Some of the bottlenecks that we're facing right now are:
- Picking the right type of cells,
- The media in which the cells grow, and
- The scale-up in the form of bioreactors.
I'll touch on each of these bottlenecks individually.
The Cells
I'll start off with the cells. Some of the traits that we are looking for within the cells are being fast and easy to grow, long lasting and flexible. I want to jump in on this a little bit. These are four different types of cells that we are able to currently grow in the lab and use to make meat.
We have access to embryonic stem cells that we can use to make several different meat types. We can also use mesenchymal stem cells; those are stem cells that are found naturally within adult bodies. So if you have an issue with taking embryonic stem cells to create meat, we can instead take biopsies, take cell types from individual adults and make meat that way. Additionally, we can take cells from individual cell types such as satellite cells, which is kind of like a baby muscle cell. Satellite cells aren't as immature as stem cells, but they're still pretty young, so we can kind of train them to do something else if we want to.

And then, additionally, we can actually do something called induced pluripotency where we can take theoretically any type of cell: We can take a feather, we can take a hair follicle, we can take a skin scraping or we can take a cheek swab, and theoretically, we can turn those into stem cells, and then in turn we can turn them into any cell type that we want. I'm saying "in theory" a lot because we haven't been able to take any cell out there and turn it into any cell that we want to, but we have been able to take a lot of different cell types and turn them into specific cell types of interest. I think that's a really exciting area of research that needs further exploration.
The next thing that I want to talk about is the ability for us to avoid continuously harvesting new cells from animals. So if we want to be able to take these cells and have them continuously grow without needing to collect new ones from animals over and over again, we need to start thinking about how we can keep these cells in a state where they are technically immortal. So with that I want to get into a little bit of cellular anatomy, so bear with me, and I'll walk you through this slide a little bit.
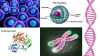
Here we have our cells again. This little green sphere here is what I like to call the cell brain, also known as the nucleus. Within the nucleus is all of the genetic information that is necessary to take an individual cell and turn that into billions of cells that make up something like a human being. That genetic information is also known as DNA, and DNA is usually wrapped up in a form of something called chromatin, which is what we are seeing here in that little X shape. And what happens is eventually this chromatin, as cells divide and the chromatin is also copied and passed onto future generations of cells, over time, about 60 cell divisions in, we hit something called a Hayflick limit.
What happens is as our cells divide, the machinery that's required for the DNA to be replicated isn't 100% efficient. So the ends of the chromosomes actually get worn down a little bit with every cell division. If you see on the chromatin structure, the ends are lime green. I don't think that chromatin is actually hot pink and lime green. I haven't personally seen real chromatin before; it's very small, so I think that this is just a visual. But the lime green is something that's called a telomere, and what those do is they serve as a buffer during every cellular division. But, again, over time this cellular division will eventually wear down all of the telomeres, and then wear down important genes that are required for things like metabolism or telling the cell to not destroy itself, because literally if a cell doesn't know what to do it's going to self destruct.
But anyway, luckily we have been able to discover a protein called telomerase, and telomerase acts by adding telomeres back onto your chromosomes, so the more research that we do into telomerase the more that we can understand in terms of large scale meat production, but we can also look into more things like curing diseases, curing aging and understanding how cancer works. So, there's a lot of really exciting research going on with telomerase.

The next thing I want to address in terms of the types of cells that we want is flexibility. So just off the top of my head I can think of at least seven different cell types that are needed in order to make up meat. On the top we have our skin cells, we have our bone cells, we have our blood cells, fat cells, cells that are responsible for making connective tissue. We also have neurons, and then of course muscle. So these are all seven very different types of cells. And it would be ideal if we would be able to take a cell type, really understand how to grow it in a controlled setting, and then also be able to get these cells to differentiate and change into all of these different cell types. And that's something that has already been shown to be doable. We can do it through media additives. We can even do it through the architecture of the scaffolding that we're putting the cells on. And additionally we can do that through genetic editing.
The Media
With that I want to talk a little bit more about the next bottleneck, which is the media, or "cell food". The media, when you think about the role of media, the first thing is that it needs to provide an adequate environment for the cells to live in, basically tricking the cells into thinking that they're still inside an animal. We need the right pH, the right temperature, the right osmotic pressure so the cells don't explode. They like to explode if they're not happy. Media also provide a lot of vitamins and minerals, as well as lipids. A lot of cells are actually made up of lipid. Cell skin is just a lipid membrane, so lots of fats are needed in order to keep cells happy. The other thing is protein. Obviously in order for one cell to turn into two cells they are going to need building blocks to actually make another cell. And, finally, growth factors.

The cool thing is that out of all of these requirements, we've been able to use plants in order to produce four. The one hiccup, though, is growth factors. That is the main issue in terms of the bottlenecks, in terms of the cost of making meat right now. This is why a chicken nugget is going to be $400 if we're growing it instead of harvesting it from an animal.
I want to talk a little bit about where we are currently getting our growth factors as well as some of the options for where we can get them later on. Originally we were getting our growth factors from baby cows. Obviously we cannot do that, so now we've started to get them through recombinant protein production. We're replacing cows with bioreactors. You guys see the foreshadowing that I'm doing here. We are able to do something called recombinant protein production where we actually take the gene that's associated with this protein or the growth factor, put it inside of a bacterium, have these bacteria grow up very fast and at very high yields, and then harvest the proteins from the bacteria. But there are other options as well. We can explore plant based growth factors or analogues. We might even be able to use chemical synthesis of the proteins that are found in plants in order to replicate these growth factors.
And we can also isolate growth factors from cells that naturally produce them in the body, which is where we're actually getting them from when we harvest blood from baby cows. Rather than making a cow we can just grow the cells that are responsible for growing the growth factors to begin with.
There is one other option that I want to explore with you, and in doing so first we need to walk through the role of a growth factor.

The growth factor serves as a form of a signal, so in this demonstration it's what the ligand is representing; it's produced and released by another cell. It then binds to the target cell's cell surface receptor, and then when that happens it triggers an intracellular pathway, and in response to this pathway we get a genetic transcription change. That, in turn, is what causes the growth. What I'm exploring right now is this bit right here, this genetic transcription, and trying to really cut out all of the middle men. Instead of relying on these growth factors in order to get the cell to do what I want, which is grow, I'm actually just designing a gene sequence that allows the cell, assuming that it has all the nutrients that it needs, to just continuously grow until we take it out of the environment that it's not happy in anymore.

I'm currently working on that at Harvard Medical School with Dr. Church, who, by the way, I met at EA Global last year. He was giving a talk and said that he was interested in exploring clean meat, so I made a beeline to him right after the talk, and now I'm in his lab, so thank you EA Global. So, these are some of the tools that I'm working on right now.
The Bioreactors
I'd like to end with the bioreactors. My money is on large scale suspension growth because that's currently what pharmaceutical companies are using right now. What we're looking for is something that's energy efficient, sterile, easy to use, and obviously scalable. So far we are able to get muscle cells to grow in suspension, so all we're needing to do now is tweak that. In terms of energy efficiency the first thing that I saw when I Googled solar-powered bioreactor was this baby right here. Obviously we need a little bit of work to go before we have that happening. But in terms of sterility, again this is something that biopharmaceutical industries are using constantly. This is only a 2,000 liter reactor. They've gotten up to 400,000 liters, so they're already doing a lot of the work for us.

And in terms of easy to use, I have personally also already used one, so if I can do it you guys can do it too. If you know how to use a computer, and you know how to work a hose, and you know not to sneeze where you're not supposed to sneeze, you can grow meat in a bioreactor. With that, I want to leave you with this image, which is a current large scale cell production facility used for biopharmaceuticals.
It might look a little complicated, might look a little expensive, and I would agree with you. But I would say in comparison to what large scale meat production facilities look like right now, this is definitely a step up. The point is that we are already here thanks to the biopharmaceutical industry, thanks to current research that's already happening that doesn't even have any sort of relationship to clean meat.

We are part of this biological revolution that's happening right now where we can actually harness the power within genetics, within biological life to create not just meat and not just food, but we can actually create life that is more efficient, that's safer, and more humane, and I believe more altruistic than ever before.
Natalie's Talk
I want to outline some of the recent developments in consumer acceptance of clean meat. There are three things I'd like to say.
First of all, a lot of the polls, both formal and informal, are methodologically lacking and not particularly useful. That said, the second thing is that even given all of those limitations, it's clear that the number of people who are interested in consuming clean meat is very, very high, so there's a lot of potential there. And thirdly, there are ways in which we can frame clean meat to make it even more attractive to consumers and to institutions. As the name of this talk suggests, we've already made significant progress with "clean" meat as one framing step, and there are others that we're thinking about and researching.
Polls
First, on to the existing informal polls. These go back to 2012, which I believe is the equivalent of millennia in clean meat years, but even in those early days, in The Guardian at least, around 70% of people said they would eat lab-grown meat. Clean meat, of course, ultimately will not be "lab-grown" meat. I think the 2014 poll shows something that was lacking from earlier polls in The Guardian in that it actually asked people about how they felt about clean meat as an alternative to conventional factory farmed meat, rather than whether they would eat it in a sort of futuristic or hypothetical setting. The Mirror, hopefully not too reflective of British society standards, was also fairly optimistic in its 2016 poll. I think the Sam Harris Twitter poll, another not completely formal methodology, was extremely promising. And the latest Memphis Meats Survey again looks very promising.

Of course there is a huge number of problems with these kinds of pollings. The way in which you phrase the question has a huge effect. The different options available for answers have a huge effect. There's nothing to stop people from answering the poll several times. And of course not all of these media outlets will capture everything important about public sentiment.
I'll just show you a few of my favorite Guardian headlines to underline that point:

I also want to show the way in which formal polls are reported can be just as misleading as informal polls. There was a paper from 2015, which was titled something like Educated Consumers Don't Think Clean Meat is an Ideal Solution. That is a gross misrepresentation of what the study was about. It was widely reported as demonstrating clean meat wasn't going to be particularly well accepted, but actually the 2,000 people polled in that survey were asked how we can solve the problem of the meat industry. There were a number of options such as eat less meat, eat no meat, support plant-based meat or clean meat, and participants could only make one choice. So this study really tells us nothing about how we can expect consumers to adopt clean meat.

Okay, the point that I think those earlier formal and informal polls make is that even if we take them at face value, and say okay, even if less than 50%, even if only 10% or 20% of consumers are going to be interested in adopting clean meat, that is huge. The current plant-based meat market is less than half a percent of the market for meat, and yet companies in that space are doing fantastically well. So even on the most pessimistic view of these surveys, which I think is incorrect, we have reason to be hugely optimistic.
Framings
Okay, I'll turn now to some of the things we can do to frame clean meat accurately, and to maximize the chances of consumer adoption going as well as possible. The first hurdle was really the name, and I think that's something that's now been well established and for the right reasons. "Clean meat" has been picked up by the vast majority of media outlets and has really been a very significant shift from "lab-based meat" and "cultured meat", which were confusing and misleading, towards "clean meat", which is both a nod to the clean energy sector, and emphasizes that this meat is genuinely just less dirty than the meat produced in the conditions on factory farms.

Here we have a graph by GFI, which was published I think a month or so ago, showing the rise of clean meat usage. And in terms of calling clean meat "meat", which it unquestionably is at a cellular level. Even the North American Meat Institute seems to be supporting not restricting the use of "meat" to mean conventional animal agriculture.

Moving beyond the name, in terms of how we can optimally frame clean meat more broadly, consumers seem to be more inclined to purchase clean meat when they're made aware of the environmental advantages, the human health advantages, the advantages in terms of sustainability. Framing is really important. One particularly interesting challenge that will be addressed soon is this feeling that clean meat is somehow not natural. There's been some interesting research on this.
There are a number of approaches I think might be viable going forward. One of those could be to say actually no, it's not natural, but the natural way in which we're producing meat at the moment is fraught with problems, and things that aren't natural, such as antibiotics, have been hugely beneficial. Another way could be to stress the fact that clean meat is, to a large extent, natural in that cells are growing as they naturally would. Whether that's in an animal or in a bioreactor is in some sense beside the point. There are also other framings that can challenge how people perceive the natural to be good.

Faunalytics is in partnership with GFI and funded by ACE, conducting an experiment that is ongoing at the moment to address how we should frame clean meat in a wider context.
Q&A
Question: If we're unable to get to a clean meat future, if 100 years from now, with all the work and energy that goes into it, we still can't get there, what do you think is the most likely reason that we won't get there?
Marie: I honestly think that the biology is there. The only reason why we could have any sort of hardship in terms of not getting there physically would probably be due to social issues, whether it be regulations put in place, or laws put in place to make sure that it doesn't happen, considering who we have fighting against us. But the biology, in my opinion, is there and ready, so it would be social issues: haters.
Question: Speaking of possible haters, I was surprised to see the acceptance, seemingly, of using the term "meat" from the meat industry. What do you think the role of the meat industry is going to be? Are they going to adopters, facilitators, opponents, haters?
Natalie: I think there's been some encouraging evidence of late. The context of that comment from the North American Meat Institute was the US Cattleman's Association, which had put forward a petition saying we can't call clean meat "meat", and this was opposed by the institution, which contains Tyson and Cargill. I think this is promising because it suggests they see themselves as part of the protein industry, the meat production industry, and not the farming industry. So I think that's encouraging. They've both invested in Memphis Meats, so the signs are looking good, I'd say, at present.
