Editorial Note
This post is a response to the question, “what new area should Open Philanthropy consider funding?” It is submitted for the Cause Exploration prize, 2022. My answer is: interventions against antimicrobial resistance.
Open Philanthropy has already conducted a shallow investigation of antimicrobial resistance (AMR) as a cause area. However, the investigation was conducted back in 2013, and suffers from the following weaknesses:
- It is very short and thus not very thorough
- It is outdated in its stats and current efforts
- It does not look at AMR as an issue that intersects with engineered pathogens
- Therefore, it does not look at AMR as a potential contributor to global catastrophic biological risks (GCBRs)
- Its funding solutions are focused heavily on antibiotic development, which is less tractable an area for research than ever
- It does not focus much at all on surveillance as an intervention, despite this being at the forefront of prevention when it comes to many infectious diseases
These aspects of the investigation motivate me to write this new shallow investigation, which aims to fill those gaps above and re-evaluate AMR as a potential cause area. Particularly when we see AMR as potentially relevant in the design of engineered pathogens and therefore as a potential contributor to GCBRs, we may see it as a much larger problem than initially expected, and with different appropriate solutions (e.g., surveillance) than those already advocated.
Disclaimer on epistemic status: I am an early career researcher trained in bioethics and beginning to work on the ethics of antimicrobial resistance policy. I am not an expert on antimicrobial resistance as such and am not familiar with this kind of desk research. This shallow investigation took me five days of desk research, reading major reports in the field. I have engaged in minimal interpretation of the facts and statistics presented here to avoid any possible misrepresentation.
Key Take Aways
- AMR is an important cause area for health and wellbeing. It currently causes around 1.27 million deaths per year (47.9 million DALYs) and is associated with 4.95 million deaths per year (192 million DALYs).[i] It killed more people that malaria or HIV/AIDs in 2019.[ii]
- Averting all deaths and DALYs from AMR is not feasible, but even with only a 50% success rate on AMR interventions, investing USD 2 per person per year in Europe, North America and Australia would save three out of four deaths from AMR.[iii]
- AMR is forecast to kill up to 10 million people per year in 2050 if not controlled.[iv]
- AMR is an important cause area when considering economic costs. Antimicrobial effectiveness is estimated to be worth USD 20-54 trillion (in constant 2007 dollars).[v]
- Antimicrobial resistance is forecast to cost more than USD 3.4 trillion globally, annually by 2030, and up to USD 6.1 trillion annually by 2050.[vi]
- Investment in AMR interventions is cost-effective: intervention packages pay for themselves after 1 year and the present net value of investing in containing AMR is USD 9.8 trillion – USD 26.8 trillion.[vii]
- Investment at these levels in Europe, North America and Australia produces a return of USD 1.5 for every dollar spent after the first year.[viii]
- AMR is a tractable cause area. Interventions for antimicrobial drug development are less likely to be productive, but surveillance, advocacy, and stewardship programmes can be. In particular, improving surveillance for early warning of outbreaks involves only simple capacity-building in already-operational laboratories in many LMICs that currently lack some additional technological needs, personnel time, and training.[ix] Examples of effective surveillance networks already exist.
- AMR is a neglected cause area. There is currently around USD 1.4 billion spent on R&D in AMR annually.[x] It’s likely that less is spent on interventions like surveillance, when we consider that one of the flagship funders in surveillance, the UK Fleming fund, has a total funding budget of £265 million (approx. USD 323 million).[xi]
- A new aspect of AMR that needs further consideration is how it intersects with global catastrophic biological risks from artificial pathogens. If a pathogen with pandemic potential were engineered to be drug-resistant, then it might kill many more people. The importance of this cause area, then, may need to be re-evaluated.
- Unfortunately, there is almost no discussion that I can find on how much worse drug-resistance makes an engineered pathogen.
- I am uncertain about several aspects of the point above.
- First, tractability, as many measures have different levels of success in different areas of the world, and much depends on capacity-building that has not yet occurred.
- Second, neglectedness. I found it very difficult to get data to estimate the total funding of AMR as a cause area, and have relied instead on estimates only of the amount spent on R&D annually.
- Third, intersections with artificial pathogens. There is almost nothing available on this but reading the literature on artificial pathogens more broadly suggests that a cautious approach regarding preparations for such pathogens is valuable.
Background
Note: Skip this if you are already familiar with the problem of AMR and possible interventions to address it.
What is the problem?
Antimicrobial resistance (AMR) is when microorganisms adapt mechanisms to resist drugs designed to kill them. This means that when we are faced with a pathogen causing serious illness (often in hospital settings and factory farming settings), the drugs that would usually cure the host are ineffective. The problem is one that is growing in size, with the UN Interagency Coordination Group on Antimicrobial Resistance (IACG) predicting up to 10 million deaths caused by AMR each year by 2050, in the worst-case scenario.[xii] This number may severely underestimate the potential impact of AMR, however, when we consider how resistance mechanisms may not only develop naturally, but also be incorporated into artificial pathogens by malevolent agents. Even not counting the risk of drug-resistance being used to increase the threat from engineered pathogens, AMR killed more people than malaria or HIV/AIDs in 2019,[xiii] and Open Philanthropy has seen malaria interventions as effective and appropriate investments in the past.
One of the reasons why AMR is not commonly thought to be a catastrophic risk is that it is limited due to the fitness cost (and resulting reduced virulence) that is often associated with resistance genes in antibiotic-free environments. In that sense, resistance and virulence (at least used to) naturally trade-off against each other. However, as the problem worsens, it becomes less comparatively costly for pathogens to have resistance genes, and so the population evolves to carry more of them. The trade-off no longer occurs in the same way. There are a number of mutation and selection mechanisms which exacerbate this problem by increasing pathogen virulence at the same time as resistance.[xiv] So, we might think there is an increasing risk of both artificial and natural antimicrobial-resistant, virulent epidemics/pandemics.
All of this is to say that if it is not sufficiently well-addressed in the coming decades, AMR could cause significant health and economic costs, with global spread, by 2050.
What are possible interventions?
Most of the possible interventions against AMR that have been most discussed relate to the development of new antimicrobials and alternative therapies. Efforts to de-link research into new effective antimicrobials and sales aim to incentivise innovation in the pharmaceutical industry. The previous shallow investigation of AMR by Open Philanthropy discussed this intervention.[xv] However, developing new antimicrobials is no longer low-hanging fruit. It becomes more difficult to develop them, the more resistance mechanisms that common pathogens develop. As such, there has been a push recently for funding efforts to focus more squarely on antimicrobial stewardship, diagnostics, alternative therapies, and surveillance.[xvi]
New, promising interventions might include funding livestock insurance programs to reduce the need for farmers to use antimicrobials as preventative measures (although, there is some uncertainty as to the effects of livestock antimicrobial use on human health).[xvii] Another might be funding capacity-building initiatives in surveillance and diagnostics in countries that have thus far struggled to act on established National Antimicrobial Resistance Action Plans, among others. Reports by international bodies like the IACG and World Bank note that funding is still inadequate for action on recommended measures.[xviii]
Importance
Health/Wellbeing Importance
At a glance
- In 2019, AMR was associated with 4.95 million deaths, of which it directly caused 1.27 million deaths worldwide.[xix]
- The same study calculated DALYs from their global AMR morbidity and mortality numbers, estimating 192 million and 47.9 million DALYs associated with and attributable to AMR, respectively.
- It killed more people than malaria or HIV/AIDs that year, according to a comprehensive study published in the Lancet earlier this year.[xx]
- If all of the drug-resistant infections were replaced with drug-susceptible infections, the authors note that the deaths and DALYs directly caused by AMR (that is, the infections people had being drug-resistant, not drug-susceptible) could have been averted. The Lancet study authors further note that some interventions against AMR focus not on ensuring infections are drug-susceptible, but on averting infections themselves, which, if successful, would result in a reduction in the AMR-associated deaths and DALYs.
Details
The WHO has claimed AMR to be one of the top ten current threats global health for humanity.[xxi] It sits alongside tackling health inequalities, reducing incidence of non-communicable diseases, and building global solidarity for health security in the WHO’s 2021 priorities.[xxii] Their concern may be unsurprising, given that in some LMICs, drug resistance rates have reached 80-90% for some antibiotic-bacterium combinations.[xxiii] The effects of resistance are severe, with many and mounting number of deaths attributable to AMR per year.
When we consider who suffers the most from AMR, the results show that young children are particularly affected, with one in five deaths in children under 5 years old currently attributable to AMR. Geographically, the most bacterial AMR-associated deaths occur in Western sub-Saharan Africa, where there are currently 27.3 deaths per 100,000 attributable to bacterial AMR, and the least number occur in Australasia, with 6.5 deaths per 100,000. Looking to the near-term future, the rate of AMR is forecast to increase by 4-7 times more in LMICs than in OECD countries between 2018-2030.[xxiv] The global distribution of bacterial AMR-associated deaths by region is shown in the below chart from the Lancet study (Figure 1 my label).[xxv]
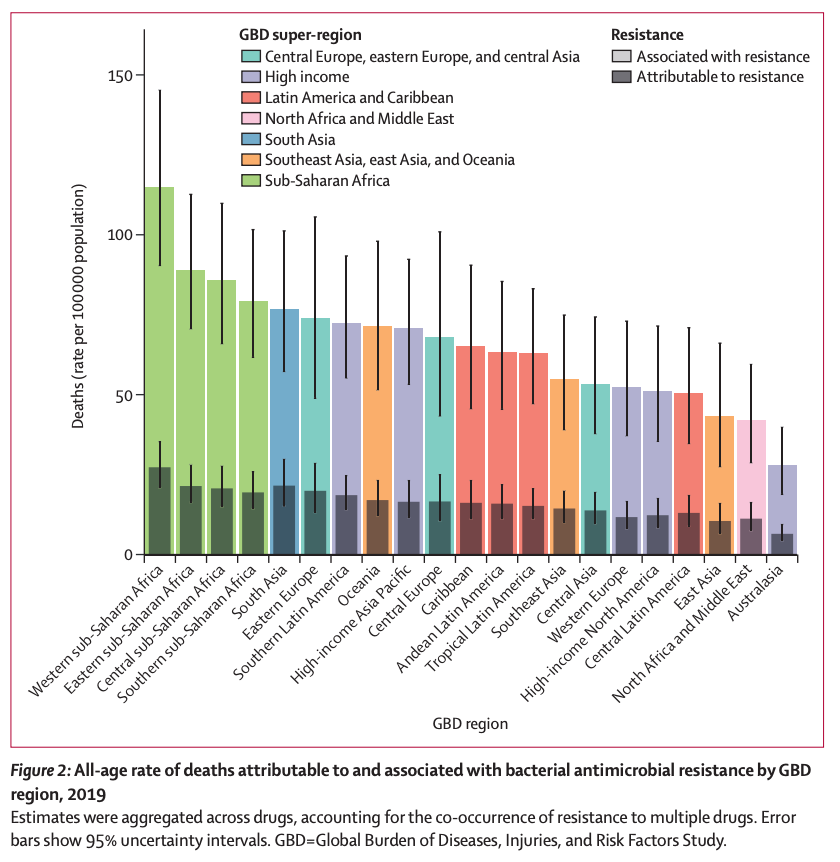
Figure 1: Source: Murray et al. 2022.[xxvi]
The importance of this is that deaths, suffering and economic losses that occur more in LMICs and more among young people may be more valuably prevented according to Open Philanthropy’s marginal benefit measure of scale than these events among the (older) population of HICs.
What’s more, due to increased prescription of antimicrobials during the COVID-19 pandemic, we may have accelerated the development of resistance in more hospital-acquired infections.[xxvii] One study found that antibiotic prescription occurred in 70% of COVID-19 cases worldwide, when only 10% had bacterial or fungal co-infections that might have been usefully treated by the antibiotics.[xxviii] Furthermore, a shift in focus from AMR projects to combatting COVID-19 stalled at least one major project, ACORN, which aims to establish an AMR surveillance network across LMICs. Hospitals hoping to implement surveillance in Asia and Africa were forced to suspend their efforts due to resource redistribution to preparation and response to the COVID-19 pandemic.[xxix]
To illustrate the importance of a particular drug-resistant pathogen, consider the global distribution of carbapenem-resistant A. baumannii (which is on the WHO’s critical priority list, and causes infections in blood, urinary tract and lungs). Whilst much of the world displays high levels of resistance, there is still some hope that in other areas (North America, sub-Saharan Africa, Australiasia) these levels can be kept low with adequate surveillance and containment measures. This is shown below (Figure 2 my label):
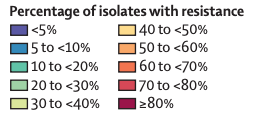
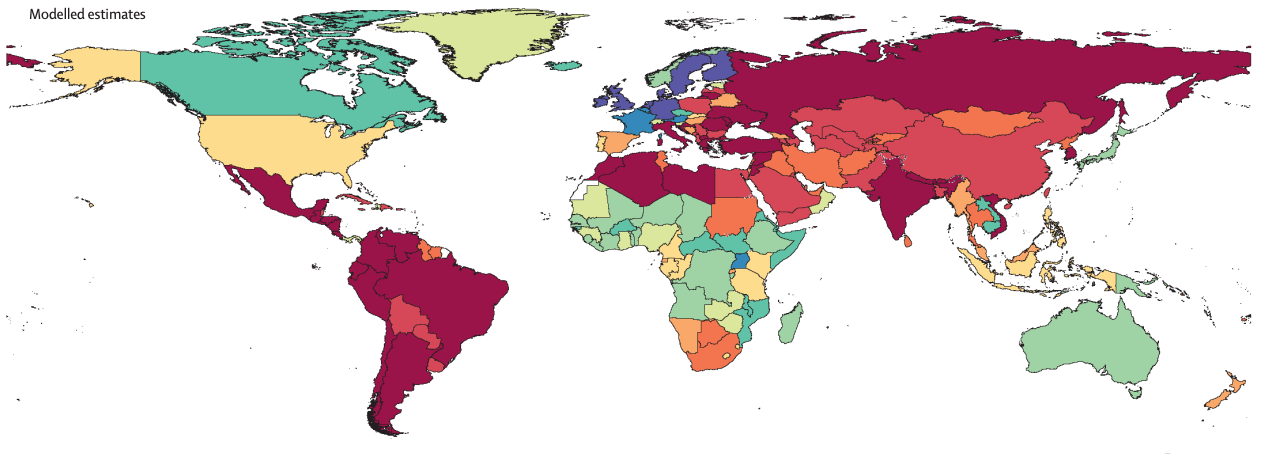
Figure 2: A. baumannii global drug resistance and susceptibility, modelled estimates for countries without data. Source: Murray et al. 2022.[xxx]
A. baumannii was associated with over 400,000 deaths in 2019, and was associated with a significantly higher risk of mortality compared to drug-susceptible A. baumannii, with an odds ratio of 2.22 (crude).[xxxi] Given this odds ratio, keeping the pathogen drug-susceptible in other areas of the world may save many lives. This case study aims to examine a particular pathogen and its course toward causing significantly higher mortality due to its drug resistance, and how this impact may be spread across the world.
Economic Importance
At a glance
Key messages on economic costs of AMR from the World Bank report:
- Antimicrobial effectiveness is an asset worth USD 20-54 trillion (in constant 2007 dollars).[xxxii]
- In a scenario where most/all of the threat of AMR was not averted, simulated global losses exceed USD 3.4 trillion annually by 2030, up to USD 6.1 trillion annually by 2050.
- Even with the threat of AMR mostly averted, simulated global losses are still 1/3 of the high-AMR scenario, exceeding USD 1 trillion annually after 2030, up to USD 2 trillion annually by 2050.
- For context, whilst the COVID-19 pandemic is forecast by the IMF to result in global losses of a little over USD 12.5 trillion, this is a total amount across the 2-3 years of the pandemic’s effects,[xxxiii] whereas the losses from AMR add as its effects grow year on year.
- The spread of these economic costs is less equal than a single global pandemic, with more of the costs being borne by LMICs than HICs, resulting in a growing income gap between LMICs and HICs.
When considering the stats above, note that the models used by the World Bank considered only shocks to the labour supply and livestock productivity, which is considered a conservative approach that underestimates the full effects of AMR.
Details
The IACG says, “The cost of AMR to national economies and their health systems is significant as it affects productivity of patients or their caretakers through prolonged hospital stays and the need for more expensive and intensive care.”[xxxiv] The IACG estimates this could amount to annual economic damage comparable to the 2008-2009 financial crisis in the event of uncontrolled AMR. They term this economic impact “catastrophic.” Whilst uncontrolled AMR may not be the most likely scenario, we should still be concerned. The World Bank has developed estimates not based on a worst-case scenario, but on one with high impact from AMR, and the estimates still reflect that up to 24 million people could come to face extreme poverty by 2030 due to AMR, mainly in LMICs.[xxxv] The World Bank’s 2017 report simulated the economic effects of AMR through 2017-2050 if adequate measures are not taken to contain AMR. Its simulations, combined with cost-effectiveness analyses, showed that “putting resources into AMR containment now is one of the highest-yield investments countries can make.”[xxxvi]
The Figure (3 my label) below illustrates the global economic losses associated with both high-AMR-impact and low-AMR-impact scenarios in the year of 2050 (not counting losses in years before then), and contrasts these with the effects of the global financial crisis of 2008-2009.
Figure 3: Source: The World Bank Group 2017.[xxxvii]
AMR in Artificial Pathogens
At a glance
- GCBRs may pose significant threat of human extinction within the next century. Drug-resistance could feature as a characteristic of a more effective engineered pathogen that might constitute a GCBR.
- This has not been much discussed at all in the literature, but given that, for instance, people with Multi-drug resistant S. aureus are 64% more likely to die than people with drug-susceptible S. aureus,[xxxviii] we might be concerned about how much worse a drug-resistant engineered pathogen might be, and how measures like surveillance for early warning on outbreaks would be effective against this threat, too.
Details
None of the exploration of the importance of AMR (health and wellbeing-related or economic importance) up to this point has taken account of the fact that drug resistance may pose a threat if used as part of an engineered pathogen. Engineered pathogens are among the most worrying GCBRs that the world may face in the coming century, according to some analyses.[xxxix] (See Figure below.) Although experts note that AMR by itself (even uncontrolled) would not constitute a GCBR, it is also discussed in the cited 80,000 hours page as a direction in which biological weapons have been developed in the past, and that could form a part of future engineered pathogens. Indeed, work has already been done examining how particular pathogens been made drug resistant as part of their development as biological weapons (for caution I will not cite those articles here). Nevertheless, the use of ‘designer genes’ for drug resistance has been a concern in the scientific community for a couple of decades.[xl]

Figure 3: Source: Lewis 2020.[xli]
As the figure illustrates, there may be a risk of 2% that human extinction will result from an engineered pandemic, with estimates given for occurrence within the next century. (Note that this table comes with an indication that its accuracy should be taken lightly as although it arises from a survey of experts, judgements are highly subjective and variable. That said, these numbers appear to be the most up-to-date estimates from experts that are available.) Yet, despite this being an area of significant concern, discussions of AMR that consider the risks it may pose as part of an engineered pathogen are almost impossible to find—at least for me, for the purposes of conducting this investigation. This may be because:
- These discussions pose security concerns;
- AMR has not yet been adequately considered in terms of the risks it poses as a component of an engineered pathogen; or
- It is already assumed to be adequately covered within the literature on engineered pathogens (as opposed to AMR) without being discussed explicitly.
Due to this gap in the literature, for now, it is hard to quantify the risk that AMR may pose as part of an engineered pathogen. To give it a shot, I expect that one could consider how many people a pathogen with pandemic potential that might be further engineered and released as an artificial pandemic (like smallpox, in its unaltered form infecting 10 to 15 million people per year and killing 5 million[xlii]) might look like if it were, as part of its engineering, made multi-drug resistant (like MRSA, which kills patients 64% more often than drug-susceptible S. aureus[xliii]). The ability to engineer in multi-drug resistance genes and change the lethality of a pathogen like that may significantly increase the risk of GCBRs in the coming century, if not only the genes, but the technology to engineer these changes continues to be increasingly available and accessible.[xliv]
Another complicating factor is that it remains difficult, according to some analyses including Open Philanthropy’s previous research,[xlv] to quantify the importance of measures to avert engineered pandemics. I thus leave aside further discussion but raise this as an uncertainty that really needs addressing.
Tractability
At a glance
Overall, it seems like investment in containing AMR is extremely cost-effective. This is backed up by reports from the World Bank[xlvi] and the OECD report ‘Stemming the Superbug Tide’ from 2018.[xlvii]
Key messages on investment to contain AMR from the World Bank report:
- AMR containment measures would cost around USD 9 billion annually in LMICs. (Compare this to the amount invested globally in responding to HIV/AIDS in 2018, USD 19 billion.[xlviii])
- The net present value of investing in containing AMR is USD 9.8 trillion – USD 26.8 trillion on the assumption that just 50% of AMR costs will be avoided by strong containment efforts between 2017-2050.
Key messages on investment to contain AMR from the OECD report:
- In Europe, North America and Australia, effective AMR management packages would cost around USD 2 per person per year to save three out of four deaths attributable to AMR.
- This investment would produce such great benefits that it would pay for itself in only a year, going on to save an estimated USD 4.8 billion per year in these countries alone (a return of USD 1.5 for every dollar spent after the first year)[xlix]
Intervention case studies from OECD report[l]:
- A package comprising hospital interventions would avert around 1.3 million DALYs, result in around 55,000 life years saved, and result in an annual average net saving (i.e., after accounting for the implementation cost of each intervention) of USD PPP 4.1 per capita across the 33 countries (OECD countries + EU28 countries) included.
- A package consisting of community actions would avert around 0.4 million DALYs, result in around 14,000 life years saved, and result in an annual average net saving of USD PPP 0.9 per capita across the 33 countries included.
- A mixed intervention package would avert around 1.1 million DALYs, result in around 47,000 life years saved, and result in an annual average net saving of USD PPP 3 per capita across the 33 countries included.
Details
Contributing to tractability, there is plenty of advice available in the AMR sphere. Public attention on AMR has been developed through a number of international groups (listed below), but there is still plenty of room for funding to fulfil the recommendations offered by these groups.
Research and Advice - International Groups on AMR:
- World Health Organisation: Raises awareness, sets priorities internationally, etc.
- UN Interagency Coordination Group on AMR (IACG): Performs research into antimicrobial resistance and sets priorities and recommendations for UN member countries.[li]
- Codex Alimentarius Commission: A WHO-FAO agency that sets standards on food safety and quality for consumer health, including levels of antibiotics in food.[lii]
- Global Leaders Group on Antimicrobial Resistance: Developed out of a recommendation contained in the IACG report. Consists of experts on AMR from UN member countries aiming to offer advice and advocacy on the AMR agenda.
- Global Antibiotic Research and Development Partnership (GARDP): Not-for-profit organisation developing new antimicrobials and alternative therapies, with a focus on sustainable and fair access.[liii]
- TB Alliance: Not-for-profit organisation that conducts and coordinates research into new, affordable drugs to treat (resistant) tuberculosis.[liv]
- CEPI: Partnership between public, private and civil society sectors to develop vaccines to prevent future epidemics.[lv]
- Others I didn’t manage to find.
Returning to the key messages introduced at the start of this section, despite the OECD report’s focus primarily on HICs, the World Bank report’s emphasis on the majority of the AMR impact befalling residents of LMICs indicates that cost effectiveness may be particularly high under Open Philanthropy’s judgement relating to marginal value of income to someone with a baseline income of USD 50,000, given that much of the counterfactual economic benefit of intervening in AMR will befall the residents of LMICs to whom additional income is more marginally beneficial than for residents of HICs with higher incomes.[lvi]
More specifically, we can look at evaluations of particular possible interventions to contain AMR, as examined in the example interventions list offered above. Whilst some of the interventions evaluated below may be more appropriately publicly funded, there is some room for philanthropic support, particularly when it comes to capacity-building through laboratory funding, research, and advocacy, as may feature particularly in the latter two packages discussed above. The hospital package above includes improved hand hygiene, stewardship programmes and enhanced environmental hygiene in health care settings. The community action package includes delayed prescriptions, mass media campaigns and use of rapid diagnostic tests. The mixed package includes stewardship programmes, enhanced environmental hygiene, mass media campaigns, and use of rapid diagnostic tests.
Another focused area for potential philanthropic intervention might be surveillance, specifically. If it does turn out that AMR should be considered as part of the threat of engineered pathogens, then surveillance may be particularly important and doubly useful for reducing both risk from AMR and risk of the spread of an engineered pathogen outbreak. The map below shows the current state of AMR surveillance networks. For many countries there’s either no data or only a national network. More regional and national networks are needed to effectively track patterns of resistance and develop informed policies to react effectively to outbreaks. The World Bank report states that in areas where there are already established effective clinical laboratories, the cost of introducing AMR surveillance is small, only involving an add-on of some extra technologies, personnel time, and training (e.g., capacity for antibiotic susceptibility testing, infrastructure, instrumentation, resource availability, quality control measures).
For illustration of the current distribution of regional (coloured) and national (red dots) AMR surveillance networks, see the figure below (Figure 4, my label).
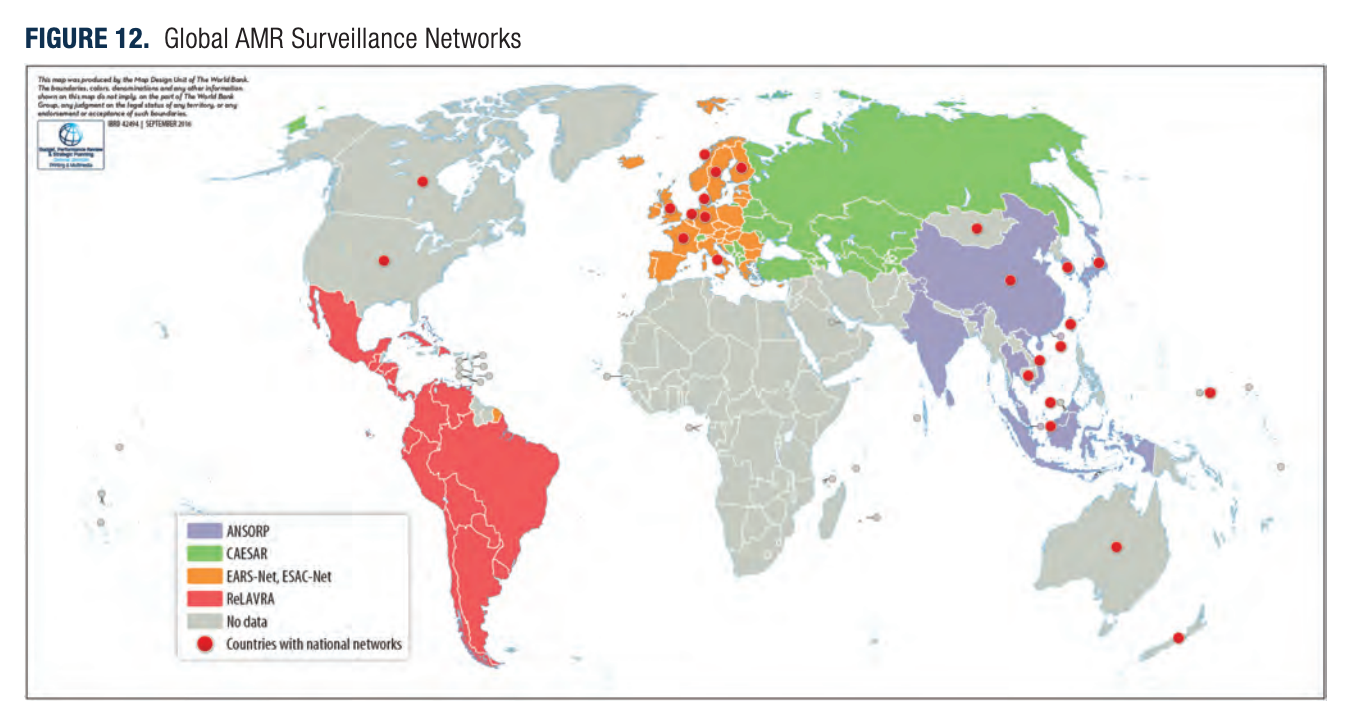
Figure 4: Source: World Bank 2017.[lvii]
Neglectedness
At a glance
There is already some funding in this area, mostly from international public and not-for-profit private groups, detailed in the list below. However, if the reports I have read are to be believed, it appears there is more work that funders can contribute to.
Funding - International Groups on AMR:
- Combatting Antibiotic-Resistant Bacteria (CARB-X): Funder that aims to accelerate antibiotic innovation by investing in scientific projects on new antibiotics and alternative therapies.[lviii] USD 396 million invested.
- Joint-Programming Initiative on AMR (JPI-AMR): European organisation that coordinates national public investments of member countries to reduce the burden of AMR through environmental, medical, surveillance and diagnostics interventions.[lix] USD 125 million invested.
- Antimicrobial Resistance Multi Partner Trust Fund (AMR MPTF): UN agency that funds interventions to address AMR through a one-health approach.[lx] USD 10 million invested.
- AMR Action Fund: Collaboration between pharmaceuticals and research funders that funds the development of new antibiotics.[lxi] USD 1 billion expected to be invested.
- Global AMR Innovation Fund (GAMRIF): UK aid fund that supports research into diverse projects to contain AMR, particularly focussed at LMICs.[lxii] £20 million (approx. USD 24 million) invested by UK and Chinese governments.
- Fleming Fund: UK public-private partnership funding research and capacity-building to gather AMR data in LMICs.[lxiii] £265 million (approx. USD 323 million) budgeted.
- Others I didn’t manage to find or didn’t list, particularly the large research funders who fund many different kinds of interventions (e.g. ERC, Wellcome Trust, etc.), for whom it would be difficult to section out their AMR-associated funding from totals.
Putting together these funding sources, my estimate for total global AMR intervention funding from funders targeted specifically to funding AMR interventions and R&D adds up to around USD 1.9 billion, between already invested funds and budgets committed to investment in the near-term future.
This value clearly massively underestimates the actual value, however. The Global AMR R&D Hub estimates around a total of USD 1.4 billion was invested annually just in research and development in the AMR space in 2017 and 2018, not counting funding actual interventions against AMR.[lxiv] Given this update, my confidence in estimating a total amount of funding in the area is very low.
Details
The literature consistently indicates that whilst there is growing awareness of AMR as a problem, there is still much needed in the way of funding. Indeed, the World Bank report states, “The private sector can contribute substantially to tackling AMR, and private-sector capacities and creativity in this area are only just beginning to be tapped.”[lxv]
The World Bank’s call for more funding is backed by the IACG, which notes that “at least 100 countries have developed National Antimicrobial Resistance Action Plans, and there is a wealth of normative guidance from the Tripartite agencies (FAO, OIE and WHO) and the Codex Alimentarius to support their implementation. But efforts to implement national action plans are currently too slow and must be accelerated.”[lxvi] The IACG holds that there is further funding needed to speed up the implementation of these plans, and that this may come both from expanding existing funding for human health through some of the organisations listed below, and from introducing new streams of funding that aim to build capacity particularly in countries that lack the infrastructure or support to implement national action plans.
Actions for Open Philanthropy
There are several areas and interventions that Open Philanthropy could consider funding when it comes to containing AMR. The list below is taken from calls for funding across the reports cited in this investigation.
1. One of the IACG’s recommendations related to the funding and capacity-building needed for National Antimicrobial Resistance Action Plans to be acted upon, particularly in LMICs. More specifically, their sub-recommendations included ensuring equitable and affordable access to existing and new antimicrobials, the implementation of standards to assess risks and impacts regarding AMR when making other investments, and strengthening and monitoring surveillance systems. They see a place for private/philanthropic funding in these areas. Open Philanthropy might consider funding AMR surveillance initiatives like the Wellcome-Pfizer collaboration on AMR surveillance.[lxvii]
2. The World Bank report emphasised livestock insurance programs claiming that philanthropic funding “will be needed to protect the livelihoods of livestock producers in low- and middle-income countries, as farmers cut antibiotic use and transition to more sustainable production models.”[lxviii] An example is the public-private partnership between the Kenyan government and Swiss Re group (and others) that insures cattle in Kenya for feed and water, to work against livestock deaths during drought and from disease.[lxix]
3. Instead of a focus on developing more antimicrobials, a shift toward alternative therapies may be more fruitful, including vaccine research. Partnerships with groups like GAVI and CEPI may help to contain AMR in the future, given the example of influenza, wherein a US study showed inappropriate antibiotic prescription in 79% of influenza patients, with estimated costs of USD 211 million annually.[lxx] It has been claimed that increased uptake of influenza vaccines could severely reduce these costs, given that there will be fewer influenza patients to begin with, and thus less need for antimicrobial stewardship decisions to be made to begin with.[lxxi]
4. If all else fails, there is still lower-hanging fruit to be picked in antimicrobial stewardship, including restriction of antibiotic use by physicians, marketing alternatives, and changing from intravenous to oral antibiotic treatments. These are indicated by this study[lxxii] to be cost-saving, low effort, low-resource measures, although updated research may be needed.
Uncertainties and Further Questions
- Interactions between AMR and climate change that might exacerbate one another.[lxxiii]
- Applicability and effectiveness of One Health approaches, given some evidence that cross-reservoir transmission may be more limited than previously thought. [lxxiv]
- To what extent should the risk of AMR being part of engineered pathogens be incorporated into an analysis of AMR as a cause area, compared to engineered pathogens/GCBRs as a cause area?
- Is it possible/useful to separate out AMR as a component of engineered pandemics such that we can address that aspect of them effectively, and focus on transmission, lethality, etc. separately?
Endnotes and References
[i] Interagency Coordination Group on AMR. 2019. No Time To Wait: Securing the future from drug-resistant infections. Accessed 04/08/22 at: https://www.who.int/docs/default-source/documents/no-time-to-wait-securing-the-future-from-drug-resistant-infections-en.pdf (pdf link)
[ii] Murray CJL, et al. 2022. Global Burden of Bacterial Antimicrobial Resistance in 2019: A systematic analysis. The Lancet 399(10325): 629-655.
[iii] OECD. 2018. Stemming the Superbug Tide: Just a few dollars more. Accessed 06/08/22 at: https://doi.org/10.1787/9789264307599-en
[iv] World Health Organization. 2021. Fact Sheet: Antimicrobial resistance. Accessed 05/08/22 at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
[v] The World Bank Group. 2017. Drug-Resistant Infections: A threat to our economic future. Accessed 05/08/22 at: https://www.worldbank.org/en/topic/health/publication/drug-resistant-infections-a-threat-to-our-economic-future
[vi] Ibid.
[vii] OECD. ‘Stemming the Superbug Tide’, op. cit. note 3.
[viii] Ibid.
[ix] Interagency Coordination Group on AMR. ‘No Time To Wait’, op. cit. note 1.
[x] Global AMR R&D Hub. 2020. The State of Public and Philanthropic Investments in AMR R&D. Accessed 09/08/22 at: https://globalamrhub.org/wp-content/uploads/2020/11/GlobalAMRHubReportDD.Nov2020.pdf
(pdf link)
[xi] Fleming Fund. Accessed 06/08/22 at: https://www.flemingfund.org/about-us/our-aims/
[xii] Interagency Coordination Group on AMR. ‘No Time To Wait’, op. cit. note 1.
[xiii] The World Bank Group. ‘Drug-Resistant Infections’, op. cit. note 5.
[xiv] Beceiro A, Tomas M, and Bou, G. 2013. Antimicrobial Resistance and Virulence: A successful or deleterious association in the bacterial world? Clinical Microbiology Reviews 26(2): 185-230.
[xv] Open Philanthropy. 2013. Antibiotic Resistance. Accessed 04/08/22 at: https://www.openphilanthropy.org/research/antibiotic-resistance/
[xvi] Interagency Coordination Group on AMR. ‘No Time To Wait’, op. cit. note 1.
[xvii] The WHO seems convinced by this link between agricultural use and human health: World Health Organization. 2017. Stop Using Antibiotics in Healthy Animals to Prevent the Spread of Antibiotic Resistance. Accessed 09/08/22 at: https://www.who.int/news/item/07-11-2017-stop-using-antibiotics-in-healthy-animals-to-prevent-the-spread-of-antibiotic-resistance. Yet, some researchers are not so convinced, see: Scott AM, et al. 2018. Is Antimicrobial Administration to Food Animals a Direct Threat to Human Health? A rapid systematic review. International Journal of Antimicrobial Agents 52(3): 316-323.
[xviii] Interagency Coordination Group on AMR. ‘No Time To Wait’, op. cit. note 1; World Health Organization. ‘Fact Sheet: Antimicrobial resistance’, op. cit. note 4.
[xix] Interagency Coordination Group on AMR. ‘No Time To Wait’, op. cit. note 1.
[xx] Murray et al. ‘Global Burden of Bacterial Antimicrobial Resistance in 2019’, op. cit. note 2.
[xxi] Ibid.
[xxii] World Health Organization. 2020. 10 Global Health Issues to Track in 2021. Accessed 05/08/22 at: https://www.who.int/news-room/spotlight/10-global-health-issues-to-track-in-2021
[xxiii] Interagency Coordination Group on AMR. ‘No Time To Wait’, op. cit. note 1.
[xxiv] OECD. ‘Stemming the Superbug Tide’, op. cit. note 3.
[xxv] Murray et al. ‘Global Burden of Bacterial Antimicrobial Resistance in 2019’, op. cit. note 2.
[xxvi] Ibid.
[xxvii] Hsu J. 2020. How Covid-19 is Accelerating the Threat of Antimicrobial Resistance. BMJ 369: m1983.
[xxviii] Rawson TM et al. 2020. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clinical Infectious Diseases 71(9): 2459-2468.
[xxix] Hsu. How Covid-19 is Accelerating the Threat of Antimicrobial Resistance, op. cit. note 27.
[xxx] Murray et al. ‘Global Burden of Bacterial Antimicrobial Resistance in 2019’, op. cit. note 2.
[xxxi] Lemos EV, et al. 2014. Carbapenem Resistance and Mortality in Patients with Acinetobacter Baumannii Infection: Systematic review and meta-analysis. Clinical Microbiology and Infection 20(5): 416-423.
[xxxii] The World Bank Group. ‘Drug-Resistant Infections’, op. cit. note 5, p. 11.
[xxxiii] Reuters. 2022. IMF sees Cost of COVID Pandemic Rising Beyond $12.5 Trillion Estimate. Accessed 09/08/22 at: https://www.reuters.com/business/imf-sees-cost-covid-pandemic-rising-beyond-125-trillion-estimate-2022-01-20/
[xxxiv] Interagency Coordination Group on AMR. ‘No Time To Wait’, op. cit. note 1, p. 1.
[xxxv] The World Bank Group. ‘Drug-Resistant Infections’, op. cit. note 5.
[xxxvi] Ibid, p. xvii.
[xxxvii] The World Bank Group. ‘Drug-Resistant Infections’, op. cit. note 5, stats and figure at pp. 18-19.
[xxxviii] World Health Organization. ‘Fact Sheet: Antimicrobial resistance’, op. cit. note 4.
[xxxix] Lewis G. 2020. Reducing Global Catastrophic Biological Risks. 80,000 Hours. Accessed 06/08/22 at: https://80000hours.org/problem-profiles/preventing-catastrophic-pandemics/full-report/
[xl] Fraser C, and Dando M. 2001. Genomics and Future Biological Weapons: The need for preventive action by the biomedical community. Nature Genetics 29: 253–256.
[xli] Lewis. 2020. ‘Reducing Global Catastrophic Biological Risks’, op. cit. note 39.
[xlii] Ochmann S and Roser M. 2018. Smallpox. Our World In Data. Accessed 09/08/2022 at: https://ourworldindata.org/smallpox#costs-of-smallpox-and-its-eradication
[xliii] World Health Organization. ‘Fact Sheet: Antimicrobial resistance’, op. cit. note 4.
[xliv] West R, and Gronvall GK. 2020. C RISPR Cautions: Biosecurity implications of gene editing. Perspectives in Biology and Medicine 63(1): 73-92.
[xlv] Open Philanthropy. 2022. Biosecurity and Pandemic Preparedness. Accessed 05/08/22 at: https://www.openphilanthropy.org/focus/biosecurity-pandemic-preparedness/
[xlvi] The World Bank Group. ‘Drug-Resistant Infections’, op. cit. note 5.
[xlvii] OECD. ‘Stemming the Superbug Tide’, op. cit. note 3, p. xx.
[xlviii] UNAIDS. 2020. Update: Investing in HIV really does pay off. Accessed 09/08/22 at: https://www.unaids.org/en/resources/presscentre/featurestories/2020/february/20200224_gow_investments
[xlix] OECD. ‘Stemming the Superbug Tide’, op. cit. note 3, pp. 17-18.
[l] OECD. ‘Stemming the Superbug Tide’, op. cit. note 3. Details on p. 19 with data tables for DALYs saved per country p. 211.
[li] World Health Organization. 2019. UN Interagency Coordination Group on Antimicrobial Resistance Presents its Report to the UN SG. Accessed 08/08/22 at: https://www.who.int/news/item/28-04-2019-un-interagency-coordination-group-on-antimicrobial-resistance-presents-its-report-to-the-un-sg
[lii] Codex Alimentarius Commission. 2022. Accessed 08/08/22 at: https://www.fao.org/fao-who-codexalimentarius/en/
[liii] Global Antibiotic Research and Development Partnership. 2022. Accessed 08/08/22 at: https://gardp.org/who-we-are/about-gardp/
[liv] TB Alliance. 2022. Accessed 08/08/22 at: https://www.tballiance.org/
[lv] CEPI. 2022. Accessed 08/08/22 at: https://cepi.net/
[lvi] For further economic evaluation, see: Baris E, Irwin A, Thiebaud A, and Evans TG. 2017. Containing Antimicrobial Resistance is a Smart Investment in Global Public Health and Wealth. AMR Control. Accessed 08/08/22 at: http://resistancecontrol.info/2017/containing-antimicrobial-resistance-is-a-smart-investment-in-global-public-health-and-wealth/
[lvii] The World Bank Group. ‘Drug-Resistant Infections’, op. cit. note 5.
[lviii] CARB-X: Combatting Antibiotic-Resistant Bacteria. Accessed 06/08/22 at: https://carb-x.org/
[lix] Joint-Programming Initiative on AMR. Accessed 06/08/22 at: https://www.jpiamr.eu/
[lx] Antimicrobial Resistance Multi Partner Trust Fund. Accessed 06/08/22 at: https://mptf.undp.org/fund/amr00
[lxi] AMR Action Fund. Accessed 06/08/22 at: https://www.amractionfund.com/?hsLang=en
[lxii] Global AMR Innovation Fund. Accessed 06/08/22 at: https://www.gov.uk/government/groups/the-global-amr-innovation-fund
[lxiii] Fleming Fund. op. cit. note 10.
[lxiv] Global AMR R&D Hub. ‘The State of Public and Philanthropic Investments in AMR R&D’, op. cit. note 9.
[lxv] The World Bank Group. ‘Drug-Resistant Infections’, op. cit. note 5, quote p. xxvii.
[lxvi] Interagency Coordination Group on AMR. ‘No Time To Wait’, op. cit. note 1, quote p. 6.
[lxvii] Pfizer. 2020. Pfizer and Wellcome Launch Surveillance Program to Combat Growing Threat of Antimicrobial Resistance in Sub-Saharan Africa. Accessed 09/08/22 at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-wellcome-launch-surveillance-program-combat
[lxviii] The World Bank Group. ‘Drug-Resistant Infections’, op. cit. note 5, p. 57.
[lxix] Swiss Re. (n.d.) Successful Kenya Livestock Insurance Program Scheme Scales Up. Accessed 09/08/22 at: https://www.swissre.com/our-business/public-sector-solutions/thought-leadership/successful-kenya-livestock-insurance-program-scheme.html
[lxx] Lipsitch M, and Siber GR. 2016. How Can Vaccines Contribute to Solving the Antimicrobial Resistance Problem? mBio 7(3): e00428-16.
[lxxi] Vanderslott S. 2017. How New Vaccines Can Help with our Antibiotic Dependence. Our World In Data. Accessed 08/08/22 at: https://ourworldindata.org/vaccines-antibiotic-dependence
[lxxii] Goff DA, et al. 2012. Is the “Low-Hanging Fruit” Worth Picking for Antimicrobial Stewardship Programs? Clinical Infectious Diseases 55(4): 587–592.
[lxxiii] MacFadden D, et al. 2018. Antibiotic Resistance Increases with Local Temperatures. Nature Climate Change 8: 510-514; United Nations Environment Program. 2017. Frontiers 2017: Emerging issues of environmental concern. Accessed 09/08/22 at: https://www.unep.org/resources/frontiers-2017-emerging-issues-environmental-concern
[lxxiv] Scott et al. Is Antimicrobial Administration to Food Animals a Direct Threat to Human Health? Op. cit. note 6.

Hi, thanks for the write up!
Your top two points:
Let's take the 2 USD per person saving 75% of deaths in EU, North America and Australia to hold across the rest of the world as that link is behind paywall.
In that case, this $2 USD * 8bn saves 75% of the 1.27m - 4.96m that currently die per year, so 0.95m - 3.7m lives saved for 16bn USD, or between 16,842 USD / life and 4,323 USD / life.
Does this seem right to you?
Hi James,
Thanks for your comment!
Your estimates sound right to me, based on the numbers you’ve used. I agree with you that it’s best to calculate based on those numbers, but I think it’s worth investigating further whether they’re on the conservative side or not.
I have the start to some thinking on that, based on the following.
First, most of the deaths from AMR do not occur in Europe, North America, and Australia (which the OECD report was focussed on). Rather, they occur in LMICs. I noted in the piece, “Looking to the near-term future, the rate of AMR is forecast to increase by 4-7 times more in LMICs than in OECD countries between 2018-2030.[xxiv]” That might be relevant to our estimates for how cost-effective intervening in AMR is, because it may be that more localised interventions are possible in LMICs, at least for some resistant pathogens. It may, then, be cheaper and still effective to intervene more locally.
Second, as the World Bank report [xlvi] noted, AMR containment measures would cost around 9 billion annually in LMICs. If you base your calculations on that figure instead, extrapolated out worldwide, the cost-effectiveness estimates don’t change too much, so it seems we don’t get over-conservative estimates from using figures for interventions in Europe, North America and Australia.
Third, one aspect that does make your estimate relatively conservative is the use of the 2019 figures. That’s the most recent data we have but recall that by 2050 we could see up to 10 million deaths per year caused by AMR. [iv]
Overall, I’m not sure what the implications are for your estimates, but just some points to bear in mind.
Thanks again for the comment!