confidence level: I am a physicist, not a biologist, so don’t take this the account of a domain level expert. But this is really basic stuff, and is very easy to verify.
Edit: I have added a few revisions and included a fact check of this post by an organic chemist. You can also read the comments on the EA forum to see Yudkowsky's response.
Recently I encountered a scientific claim about biology, made by Eliezer Yudkowsky. I searched around for the source of the claim, and found that he has been repeating versions of the claim for over a decade and a half, including in “the sequences” and his TED talk. In recent years, this claim has primarily been used as an argument for why an AGI attack would be extremely deadly. I believe this claim is factually incorrect.
The quotes:
I’m going to show the various versions of the claim I found below, with the relevant sentences bolded:
To plausibly argue that “humans” were intelligently designed, you’d have to lie about the design of the human retina, the architecture of the human brain, the proteins bound together by weak van der Waals forces instead of strong covalent bonds
-Yudkowsky discussing the flaws of evolutionary design, in “the sequences” blog post “dark side epistemology”.
It was obvious years before Nanosystems that molecular nanomachines would in fact be possible and have much higher power densities than biology. I could say, "Because proteins are held together by van der Waals forces that are much weaker than covalent bonds," to point to a reason how you could realize that after just reading Engines of Creation and before Nanosytems existed.
- Yudkowsky discussing AI interventions on the alignment forum.
A lot of the advantage of human technology is due to human technology figuring out how to use covalent bonds and metallic bonds, where biology sticks to ionic bonds and proteins held together by van der Waals forces (static cling, basically)
-Comment on a post discussing technology and AI.
Algae are tiny microns-wide solar-powered fully self-replicating factories that run on general assemblers, "ribosomes", that can replicate most other products of biology given digital instructions. This, even though the proteins are held together by van der Waals forces rather than covalent bonds, which is why algae are far less tough than diamond (as you can also make from carbon). It should not be very hard for a superintelligence to repurpose ribosomes to build better, more strongly bonded, more energy-dense tiny things that can then have a quite easy time killing everyone.
-Yudkowsky’s example scenario for how an AI could extinct humanity, on twitter
Can you build your own synthetic biology, synthetic cyborgs? Can you blow straight past that to covalently bonded equivalents of biology where instead of proteins that fold together and are held together by static cling, you have things that go down much sharper potential energy gradients and are bundled together, people have done advanced design work about this sort of thing.
-Yudkowksy’s Ted talk, again discussing AI capabilities, during the Q&A section.
I broadly endorse this reply and have mostly shifted to trying to talk about “covalently bonded” bacteria, since using the term “diamondoid” (tightly covalently bonded CHON) causes people to panic about the lack of currently known mechanosynthesis pathways for tetrahedral carbon lattices.
-Yudkowsky’s response to my recent article a few weeks ago, talking about how to refer to potential advanced nanotechnologies.
Summarising the claim
As you can see, Yudkowsky has repeated this claim several time over a time period spanning 15 years to just a few weeks ago, in very high profile contexts.
These quotes (intentionally or unintentionally) all make roughly the same argument, which I will sum up as follows:
Proteins are held together by weak van-der-waals forces, which are weak forces, akin to static cling.
In contrast, alternatives to biological proteins could utilize strong covalent bonds, and would therefore be much more powerful.
Edit: Yudkowsky has claimed in the comments that this was not the intended message of his statements. Nonetheless, this is what he ended up saying.
Although the claim is deployed in a few different contexts, I will focus this article on the context of molecular nanotechnology, where it forms part of an argument for the potential deadliness of rogue AGI.
Bond types
Let’s start off by defining four common types of bonds. These are not the only types of bonds (for example, metallic bonds are strong but will not be discussed here). Note that the following definitions are all chem 101 simplifications. The bond strengths vary depending on which atoms are bonded and a bunch of other factors, but I just want to give a general picture here.
Covalent bonds: Covalent bonds arise when atoms “share” electron pairs between them, allowing them to get closer to a complete “shell”. These bonds are very strong, typically being hundreds of kJ/mol, depending on the atoms involved.
Ionic bonds: Some atoms really want to get rid of electrons, and some atoms really want to receive extra electrons. If one of each meets, an electron will jump from one to the other, making each atom oppositely charged, so they stick together. Ionic bond strength can vary quite a bit, with variations between 170 and 1500 kj/mol.
Hydrogen bonds: These bonds occur because hydrogen only has one electron. If that one electron covalently bonds with another atom, then it’s stuck on one side of the hydrogen, so the other side is positively charged. At the same time, another nearby atom, like oxygen, might have unbonded electrons hanging out preferentially on one side. This brings the hydrogen and other atom together electrostatically, where they bond. Compared to the previous two, this is a comparatively weak bond, typically around 10 kJ/mol.
This is the name for a collection of very weak forces acting between atoms. Primarily these are a result of fluctuating polarisations on neighbouring atoms, and tend to be transient and weak. The strength varies depending on definitions, with one source telling me 0.4 to 4 kJ/mol, and wikipedia mentioning strengths as low as 0.04 kj/mol. They are generally defined by their weakness.
A definitional note: Some sources will include hydrogen bonds as a subset of Van der Waals forces, as they both count as intermolecular forces. It is more common to separate the two definitionally, such as by saying that hydrogen bonds are permanent dipoles, while Van der Waals forces are not. The main reason to do so is that hydrogen bonds are generally much stronger and more permanent than Van der Waals forces.
Edit: Talking merely about the types of bonds can be misleading when talking about material strength, as the amount of bonds also matters. A chemist told me that "long series of hydrogen bonds or even vdW forces (in things like UHMWPE fibers) can be stronger than single covalently bound strands". This is another problem with the quotes above.
Bonds in proteins
Now that we’ve got these definitions in place, let’s get to the question at hand. What forces are present in biology? The image below shows the primary structure of a protein chain.
What percentage of the bonds shown below correspond to each of the four bond types I listed above? I encourage you to take a guess now, before scrolling down.
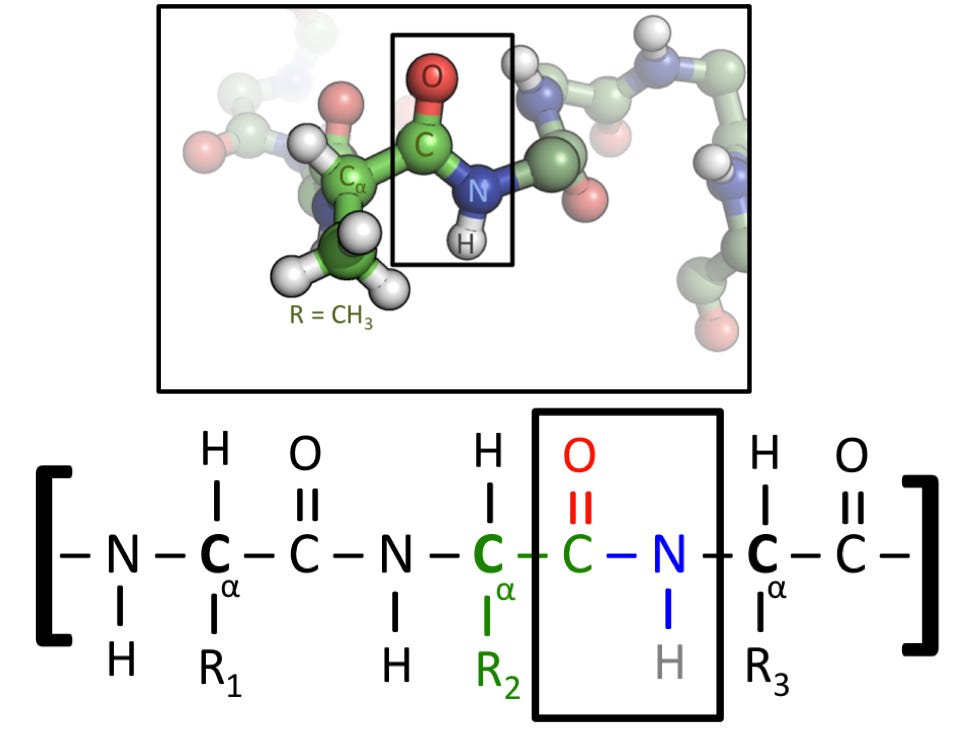
The answer is as follows:
100% of the bonds are covalent.
Okay, I’ll admit, I have played a small trick here, and to explain why, I’ll have to explain a bit more about how proteins work.
To build a protein, a ribosome will read DNA to choose from a set of 22 available amino acids, and then stitch them together like beads on a string. Each amino acid is held together almost entirely by covalent bonds, and the peptide bonds between amino acids are also covalent in nature. This initial string is called the “primary structure”. The R1, R2 and R3 in the above picture represent “side chains”, extra bits that are unique to each amino acid and can have a significant effect on it’s behavior. These side chains are also primarily (or possibly entirely?) covalent in nature.
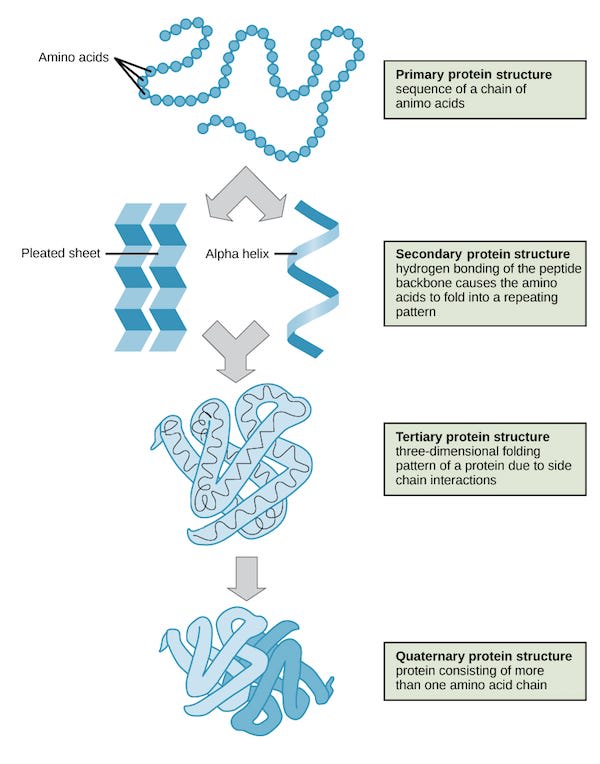
However, a long string is not a stable structure. As soon as this bead is spit out, it starts wiggling around and folding up until it’s reached a relatively stable 3d structure. The “protein folding problem” of predicting how this fold would occur was a super-hard problem, which was recently cracked using deep learning techniques by Deepminds Alphafold 2 program, which can now predict 3d structures from base structures with very high accuracy. Folding structure is often divided up into three or four regimes of structure as shown in the following image:
Okay. So we’ve established that primary structure is overwhelmingly covalent, let’s move on to the “secondary structure”. This is where the chain folds up into itself so that the “backbone” (the none side-chain part) of one section bonds with the backbone of the other. This usually takes the shape of either parallel sheets or helices, as shown in the image below.
Feel free to estimate once again, for the next image, how the bond types are divided up:
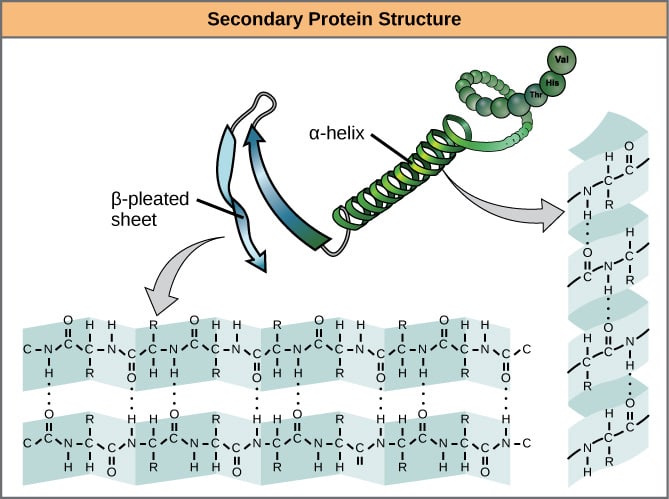
I did a rough count, and in the sheet pictured it’s roughly 90% covalent bonds. The other 10% are hydrogen bonds. It looks like a similar proportion applies in the helix, although the picture doesn’t show the other side.
Up at the tertiary level, everything becomes a lot less predictable: proteins can fold every which way, to make a huge variety of structures, which depend a lot on the individual amino acid side chains that make up the structure. The following image shows a few ways that the protein is pinned together at a higher level.
I’m not going to ask to predict the bonds this time, because I think it’s highly material dependent at this point.
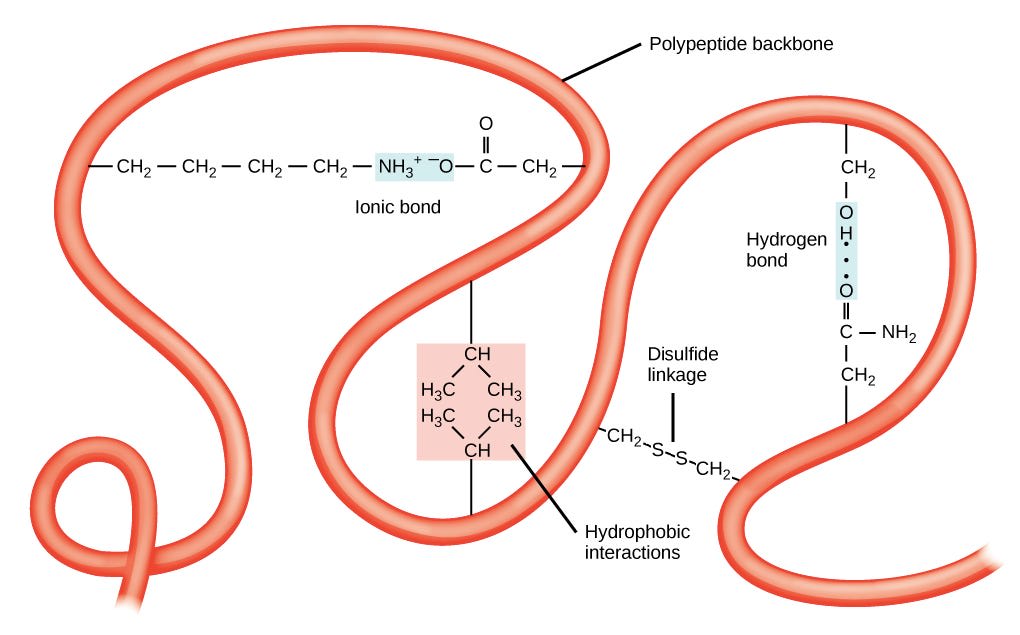
We can see that in tertiary structure, side chains of the main protein can bond to each other in a ton of different ways. We can have ionic bonds, hydrogen bonds, Van der Waals interactions, and… yep, covalent bonds, in the form of disulfide links. These can act as sort of “pins”, locking folded structures in place.
This website has a nice 3d model that lets you play around and explore the different types of bonds in a complex protein.
So at long last, we have all four types of bonds available, and the proportions of each is just gonna depend on the exact sequence of amino acids used. However, for most if not all proteins, the order of prevalence will be covalent bonds>hydrogen bonds> everything else.
To summarize, proteins are held together by a combination of bond types, primarily covalent bonds and secondarily hydrogen bonds, with other forces playing some part, depending on the protein.
Edit: I think that the previous summary was a little muddled. I would summarize it now as follows:
The primary structure of protein is held together with covalent bonds, with the secondary backbone held together by hydrogen bonds. At the tertiary level, these chains are held together by a combination of many different bonds and forces, such as hydrophobic interactions, hydrogen bonds, Van der Waals forces, and covalent bond links, all of which contribute to it's 3D structure with differing importance depending on the material.
As a result, I am now certain that the statement “proteins are held together by van der Waals forces rather than covalent bonds” is false. It’s false even if you put hydrogen bonding in the “Van der Waals forces” category, which would be misleading in this context. Nobody who knew about the actual structure of proteins would use the phrase “covalently bonded alternatives to biology”. The entire field of organic chemistry arises from carbon’s thirst for covalent bonds.
Weirdly, I’ve popped open my copy of “engines of creation”, which Yudkowsky cites for his claims, and I see nothing about Van der Waals forces or covalent bonds. In fact, Drexler spends quite a while praising the flexibility and versatility of proteins: he views custom designed protein machines as the first step on the road to nanotech. He merely notes that biological material are not as durable as metals and diamond. Of course, Drexler is not a biologist so even if he had said it, it would still have been worth fact-checking.
“Covalent bonds” were not the motivation for molecular nanotech. Rather, it was about atomically precise manufacturing: that you could avoid the wiggly randomness of biology and instead place molecules atom by atom mechanically using a system of nanoscale manipulators. The hope was that you could build a universal assembler that could build anything, and not be limited to biological materials or biological assembly techniques. This would include extremely strong structures such as diamond or pure metals, but could also be anything else that MNT proponents can dream of. If you wanted a new car, you’d just send it off to a universal assembler, which would build the entire thing from scratch in your garage.
Of course, it’s easy to dream up such things, and much harder to actually build it. As I described in a previous post, attempts to actually build such a thing have completely stalled at the first hurdle. It may just be unworkable in practicality.
Gullibility filters
I think a key danger of these sort of mistakes is that they act as a gullibility filter.
Anybody with a chemistry or biology background who hears someone confidently utter the phrase “covalently bonded equivalents to biology” will immediately have their bullshit alarm triggered, and will probably dismiss everything else you say as well. This also goes for anyone with enough skeptical instinct to google claims to ensure they have the bare minimum of scientific backing.
This isn’t an isolated incident either, by the way. See my write-up on the errors in the math in the quantum physics sequences, or the errors in economics described here.
So the people who know things, and the people who actually google things, will disproportionately come to the conclusion that EA is talking nonsense and not join, whereas the people who blindly accept any scientific sounding word they hear will just walk right in. I think this is not good for the epistemic health of a community.
Strong biology?
Moving on, what do I think of the broader points about biology being “weak”? Well it’s not like biology has slept on the idea of “make strong things”:
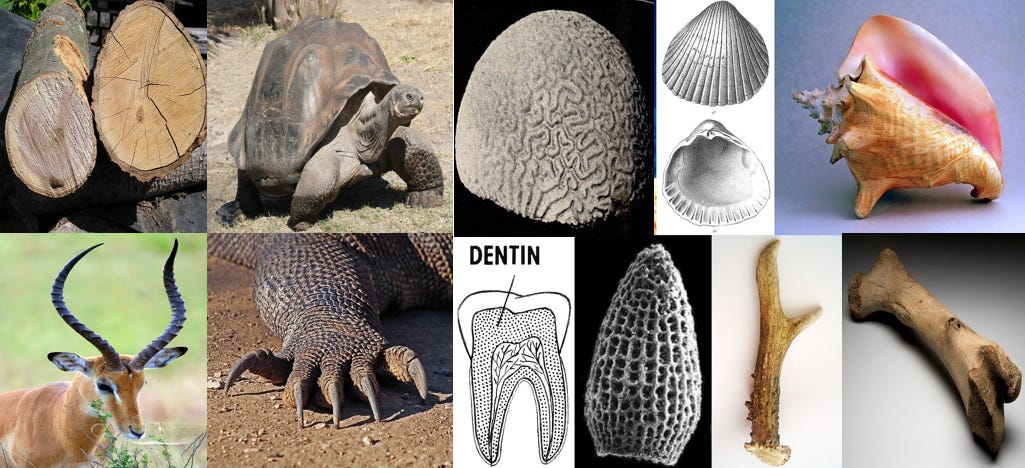
Have you heard of wood? Horns? claws, shells, bones? They all seem pretty strong to me. There are also materials with extremely high strength to weight ratios, like spider silk. Some of these are made with hard proteins such as keratin, some are made with mineralized tissue, or a combination the two.
This is not to say that a fully diamondoid based nanobot, (if such a thing is even possible) wouldn’t be stronger than these examples. Protein is not entirely covalent, which introduces weaknesses. When put into extreme conditions such as high heat, they may lose their secondary and tertiary structure and unfold back to their primary covalently bonded structure, in a process called “denaturation”. Diamond can avoid this fate as all of it’s bonds are equally strong.
My point here is merely to say that this is not a case of diamond vs “static cling”, but diamond vs wood or bone. Don't underestimate biological ingenuity!
Flexibility
My other point is this: Are we sure we even want our proteins to be strictly covalently bonded?
In my previous post, I covered the woes of trying to stick two carbon atoms to a carbon surface. The issue was this: you have to pick the atoms up and drop them in place. But the only way to pick them up, at that scale, was to covalently bond them to your transport tip, then covalently bond the atoms and the tip to the surface, and then forcibly rip the transport atom from the surface to leave .The analogy I used was that it was like trying to lay bricks when your gloves are coated in superglue.
In this scenario, the fact that covalent bonds are strong is the problem. It would actually be fantastic if you could use weak forces here: you lightly stick the atoms to your transport tip, drop it into the covalent bond spot, and then easily pull the transport tip away.
Biology pretty much does this. For example, enzymes like the one pictured below hold molecules in place through noncovalent bonding, facilitate a chemical reaction between them, and then release them.
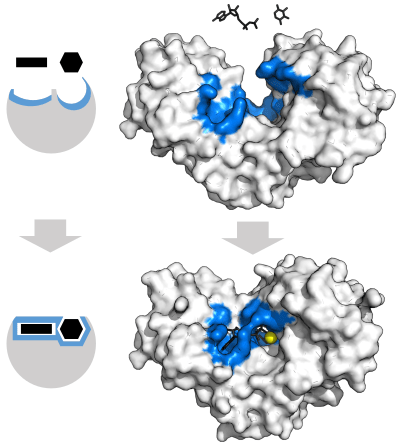
As part of the process, parts of the enzyme actually shift and mold their structures around the incoming molecules in order to better catalyse reactions. I’m not sure how easily you could replicate this using stiff strictly covalent structures. Edit: read the response by an organic chemist below for further discussion on this point.
Part of the reason that proteins are so flexible and versatile is exactly the fact that they aren’t fully welded together by covalent bonds, and thus can utilise multiple strengths of force as necessary for their purposes.
Back to the drawing board?
Imagine there was a boss and inventor who knew nothing about biology, and set out to build molecular machines. After fifty years of hard work, he rushes in and we get the following exchange:
Inventor: “EUREKA! I’ve invented a fully functioning self-replicating molecular machine. We can use this elaborate structure called DNA to encode strings of amino acids, and they will automatically fold into a virtually unlimited variety of 3 dimensional structures that can do a huge variety of microscale tasks, including replicating themselves using these incredibly complicated structures called “cells”. The possibilities are endless!”
Boss: “eh, I dunno, this design seems like weak shit. It’s not as tough as diamond and it's not entirely covalent. I reckon you should abandon this design entirely and go back to the drawing board, it can’t be that hard to build something better”.
He may be right that something better is possible. But that doesn’t mean that something better can be built quickly. The DNA/RNA model has been refined with over 3 billion years of practical trial and error experimentation on a planetary scale. Evolution may be dumb and slow, but it’s also got a ridiculous head start.
I do not expect evolution to have created the best molecular machinery it is possible to ever make, given infinite time and resources. Evolution provides constraints on how changes can occur, so if you remove these constraints, you can, almost by definition, do better. But it seems to me like the smart thing to do, if you want to build effective self replicating nanomachines quickly, is to piggyback off the natural experimentation of biology, and make even cooler and better things within the DNA framework, rather than starting from scratch and trying to reinvent the wheel. I think this is true for humans, and I think it’s also true for non-godlike AI’s.
I am very excited to see what can be accomplished in biology in the years to come. We are now in the age of Alphafold 2 and CRISPR, and I expect at least some incredibly cool stuff to come out of that. In my previous post, I highlighted DNA robots and DNA origami as impressive advances that I will keep an eye on, as we learn it’s advantages and limitations .
I think there is still a lot of room to explore what biology tells us about the potential capabilities and dangers of molecular machines, biological or otherwise. I would be very interested to hear from any biologists who have expertise in these areas.
Conclusion:
I believe that the claims about protein bonding repeatedly made by Yudkowsky are factually incorrect. Proteins are primarily held together by covalent bonds, and secondarily by hydrogen bonds, with Van der Waals forces sometimes but not always contributing. Edit: Proteins are held together by a variety of forces, with a primary structure of covalent bonds, and also contain covalent bonds at the tertiary level, including sometimes as the dominant structural force. It is true that molecular machines based on diamondoid or metal could be physically stronger that biology, but attempts to build such machines have completely stalled. Biology can still build very strong materials such as wood and bone, and the use of non-covalent bonds can actually be very useful for the flexibility and versatility of proteins such as enzymes.
Edit: a breakdown of the claims I have a problem with
I originally left this as a comment, but decided to include it as an edit in the post as well. I thought that the problems with the original quotes were self-evident, but it may be worth breaking down each quote in turn. The following are ranked from roughly most to least wrong.
human technology figuring out how to use covalent bonds and metallic bonds, where biology sticks to ionic bonds and proteins held together by van der Waals forces
This is just straight up, explicitly false. Biology does not "stick to ionic bonds and proteins". As I pointed out, biology is made up of covalent bonds at it's very core, and uses them all the time.
covalently bonded equivalents of biology where instead of proteins that fold together and are held together by static cling, you have things that go down much sharper potential energy gradients and are bundled together
The phrase "covalently bonded equivalents to biology" implicitly states that biology is not covalently bonded. This is false.
I have mostly shifted to trying to talk about “covalently bonded” bacteria
The context of this claim is that Yudkowsky is trying to come up with a new name for deadly Drexler-style nanomachines. He has chosen "covalently bonded bacteria", implying that "covalently bonded bacteria" and normal bacteria are different things. Except that's not true, because bacteria is completely full of covalent bonds.
Algae are tiny microns-wide solar-powered fully self-replicating factories that run on general assemblers, "ribosomes", that can replicate most other products of biology given digital instructions.
Okay, I just saw this one, but ribosomes are not "general" assemblers, and they cannot replicate "most other products of biology". They do literally one thing, and that is read instructions and link together amino acids to form proteins.
For the next two, let's establish the principle that if you say "X is held together by Y instead of Z", you are implicitly making the statement that "X is not held together by Z", or perhaps that "Z is irrevelant compared to Y when talking about how X is held together", or that "Y is the dominant structural force compared to Z". Otherwise you would not have used the word instead of. Would you utter the phrase "animal bodies are held together by flesh instead of skeletons?"
proteins bound together by weak van der Waals forces instead of strong covalent bonds
This implicitly makes the statement "proteins are not held together by strong covalent bonds", which is false. Or it could be saying "strong covalent bonds are irrelevant compared to van der waals forces when talking about how proteins are held together", which is also false. edit: Or it is saying that "van der waals forces are the dominant structural force in proteins", which is also false, because this is materially dependent, and some proteins have covalent disulfide links as their dominant structural force.
even though the proteins are held together by van der Waals forces rather than covalent bonds, which is why algae are far less tough than diamond
"rather than" means the same thing as "instead of", and therefore makes an implicitly false statement for the reason I said in the last quote.
proteins are held together by van der Waals forces that are much weaker than covalent bonds
Actually, this one is defensible, because it didn't use the phrase "instead of". I would still prefer more qualifying terms such "the weakest link". If this had been the only statement, I would not have written this post.
It is fairly easy to fix most of these statements. As an example, you could say something like "the 3d structure of protein contains weak links like hydrophic bonds that are easy to break apart, whereas Drexler style tech could be made from 100% densely packed strictly covalent bonds"
I appreciate the difficulty of science communication and the need for simplification, but I believe that if it is easy to avoid saying false things, you should do so.
Final edit:
An organic chemist going by "skillissuer" reached out, saying they broadly agree with the post, but had nitpicks. I thought it was interesting enough to just include what they wrote directly here:
I think that chemistry 101 classification of bonds is a tad useless here. Instead, you can go from first principles: there are things that happen when atomic orbitals overlap (covalent bonds, metallic and such), there are interactions that are mostly electrostatic in nature (ionic, dipole-dipole, quadrupole-quadrupole - important biologically as pi-stacking, also ion-quadrupole etc) and there are things that are a result of exchange interaction (van der Waals and steric repulsion). Hydrogen bonds would be a mix of dipole-dipole and van der Waals interaction. You don’t have to transfer electrons in order to have ionic interaction, most of the time in biologically relevant situations it’s proton transfer, or charges just were there previously. Hydrophobic interactions are almost entirely a solvent effect and aren’t a bond strictly speaking
In water, i’m pretty sure that proteins are mostly held by hydrogen bonds and hydrophobic interactions. EY is correct in that some proteins hold shape by mostly noncovalent interactions, but these are mostly hydrogen, ionic, hydrophobic interactions and the proteins that actually provide mechanical strength run in continuous covalent strands through entire length of them anyway (collagen, keratin). I don’t think that counting bonds and saying that something is 90% bound covalently is a meaningful metric, because long series of hydrogen bonds or even vdW forces (in things like UHMWPE fibers) can be stronger than single covalently bound strand, ie if you tried to pull out a single strand of kevlar or collagen from bulk material, above certain length you won’t pull it apart, you’d just break it because collective energy of hydrogen bonds will be greater than single covalent bond holding it together, that’s why these fibers are strong in the first place
There is another kind of flexibility that you haven’t mentioned: proteins are made out of single covalently bound strand, yes, but these aren’t straight C-C chains. Making and especially breaking C-C bonds in controlled way is hard, proteins can be just hydrolyzed at amide bonds. If protein breaks in some way, and in real world everything breaks, it can be recycled into aminoacids (+ any cofactors etc) and then put back in a pretty straightforward way; you can’t do this with diamondoids, when it breaks, it breaks hard, and you’re done unless you’re picking everything apart atom by atom which would be much harder and more energy intensive. As it happens you can buy bulk adamantane, but it’s just made in conditions where C-C bonds are weak (high temperature) and it’s preferentially formed because it’s most stable thermodynamically among its isomers (that are starting materials). Conversely, if you use weaker bonds, you can make pieces conform to some template, or to each other without breaking everything at once - this is basis of dynamic combinatorial chemistry. There’s also entire field of self-healing materials that is based almost entirely on these either noncovalent or reversible covalent bonds
As part of the process, parts of the enzyme actually shift and mold their structures around the incoming molecules in order to better catalyse reactions. I’m not sure how easily you could replicate this using stiff strictly covalent structures.
You actually don’t have to do that, and there are some small organocatalysts that are entirely covalently bonded and do the same job. However you can’t make them from from aminoacids, these don’t have secondary structure (too small) and are generally less active. The bare minimum is to provide a receptor for transition state, and you can make it work without drastic changes in conformation. You could make your catalyst as stiff as you like, and it’ll even make activity higher - but only if none of these stiff parts interfere with binding of substrates, and your options are limited. It’s often better to leave some wiggle room. Short peptides aren’t really stiff enough in ways that matter there and instead it’s secondary and tertiary structure that puts important bits in the right place

Hi, computational protein engineer and person who-thinks-biology-can-do-amazing-stuff here.
Just wanted to report that while "Proteins are like Folded Spaghetti Held Together By Static Cling" is obviously incorrect as a matter of fact, I immediately thought it was a pretty good analogy for capturing some critical and often under-appreciated aspects of the functionally important character of proteins. When I read the sentences you've quoted him saying about proteins held together by covalent bonds, I (think) I understood what he was pointing at with this and roughly agree. I absolutely did not think he was saying proteins did not contain covalent bonds, but rather that you could imagine an alternative protein-like molecular structure which had all of its key structural and functional characteristics determined by covalent bonds. I believe such a "protein" would, among other things, be predicted to function at a much wider range of temperatures than extant proteins, maintain its structure and function in many different solvents (and probably gas phase?) etc etc.
This analogy is also definitely failing to capture other important characteristics of proteins, some of which the OP mentioned and I agree with.
By the way, if I was forced to say one thing which "held together" proteins (in the sense of "is most responsible for determining the functional characteristics of a polypeptide chain) it would be tough but I might pick something I don't think anyone has mentioned: hydrophobicity/ hydrophilicity.
I'm not sure what's a truer analogy than static cling for hydrophobia as a force holding things together which the general audience has any experience with. Macroscopic experience of hydrophobia is, like, oil collecting on the surface of water, which isn't experienced as a binding force the way that static cling is.
Yea, idk. I was thinking of the quotes where you explicitly mentioned Van der Waals forces. Tbc, my preference would be to not be forced to pick a single force
Hey, thanks for replying! I explained my issues with the wording he used in a different comment. I would rather know more about what you think about the subject.
What do you mean by "protein-like" here? Like, a stitched together chain of molecules that also folds up, but has much stronger cross-links? Or like a 2d-layering system? Or the gears and manipulators that Drexler proposed and wrote up here? Do any of these sound plausible, or easily built off of regular biological systems?
Are you aware of any potential alternative designs for biology compared to the DNA/RNA approach?
Sorry if these are too many questions, I'm very interested in this subject but have reached the limit of my expertise.
Why is flesh weaker than diamond? Diamond is made of carbon-carbon bonds. Proteins also have some carbon-carbon bonds! So why should a diamond blade be able to cut skin?
I reply: Because the strength of the material is determined by its weakest link, not its strongest link. A structure of steel beams held together at the vertices by Scotch tape (and lacking other clever arrangements of mechanical advantage) has the strength of Scotch tape rather than the strength of steel.
Or: Even when the load-bearing forces holding large molecular systems together are locally covalent bonds, as in lignin (what makes wood strong), if you've got larger molecules only held together by covalent bonds at interspersed points along their edges, that's like having 10cm-diameter steel beams held together by 1cm welds. Again, barring other clever arrangements of mechanical advantage, that structure has the strength of 1cm of steel rather than 10cm of steel.
Bone is stronger than wood; it runs on a relatively stronger structure of ionic bonds, which are no locally weaker than carbon bonds in terms of attojoules of potential energy per bond. Bone is weaker than diamond, then, because... why?
Well, partially, IIUC, because calcium atoms are heavier than carbon atoms. So even if per-bond the ionic forces are strong, some of that is lost in the price you pay for including heavier atoms whose nuclei have more protons that are able to exert the stronger electrical forces making up that stronger bond.
But mainly, bone is so much weaker than diamond (on my understanding) because the carbon bonds in diamond have a regular crystal structure that locks the carbon atoms into relative angles, and in a solid diamond this crystal structure is tesselated globally. Hydroxyapatite (the crystal part of bone) also tesselates in an energetically favorable configuration; but (I could be wrong about this) it doesn't have the same local resistance to local deformation; and also, the actual hydroxyapatite crystal is assembled by other tissues that layer the ionic components into place, which means that a larger structure of bone is full of fault lines. Bone cleaves along the weaker fault line, not at its strongest point.
But then, why don't diamond bones exist already? Not just for the added strength; why make the organism look for calcium and phosphorus instead of just carbon?
The search process of evolutionary biology is not the search of engineering; natural selection can only access designs via pathways of incremental mutations that are locally advantageous, not intelligently designed simultaneous changes that compensate for each other. There were, last time I checked, only three known cases where evolutionary biology invented the freely rotating wheel. Two of those known cases are ATP synthase and the bacterial flagellum, which demonstrates that freely rotating wheels are in fact incredibly useful in biology, and are conserved when biology stumbles across them after a few hundred million years of search. But there's no use for a freely rotating wheel without a bearing and there's no use for a bearing without a freely rotating wheel, and a simultaneous dependency like that is a huge obstacle to biology, even though it's a hardly noticeable obstacle to intelligent engineering.
The entire human body, faced with a strong impact like being gored by a rhinocerous horn, will fail at its weakest point, not its strongest point. How much evolutionary advantage is there to stronger bone, if what fails first is torn muscle? How much advantage is there to an impact-resistant kidney, if most fights that destroy a kidney will kill you anyways? Evolution is not the sort of optimizer that says, "Okay, let's design an entire stronger body." (Analogously, the collection of faults that add up to "old age" is large enough that a little more age resistance in one place is not much of an advantage if other aging systems or outward accidents will soon kill you anyways.)
I don't even think we have much of a reason to believe that it'd be physically (rather than informationally) difficult to have a set of enzymes that synthesize diamond. It could just require 3 things to go right simultaneously, and so be much much harder to stumble across than tossing more hydroxyapatite to lock into place in a bone crystal. And then even if somehow evolution hit on the right set of 3 simultaneous mutations, sometime over the history of Earth, the resulting little isolated chunk of diamond probably would not be somewhere in the phenotype that had previously constituted the weakest point in a mechanical system that frequently failed. If evolution has huge difficulty inventing wheels, why expect that it could build diamond chainmail, even assuming that diamond chainmail is physically possible and could be useful to an organism that had it?
Talking to the general public is hard. The first concept I'm trying to convey to them is that there's an underlying physical, mechanical reason that flesh is weaker than diamond; and that this reason isn't that things animated by vitalic spirit, elan vital, can self-heal and self-reproduce at the cost of being weaker than the cold steel making up lifeless machines, as is the price of magic imposed by the universe to maintain game balance. This is a very natural way for humans to think; and the thing I am trying to come in and do is say, "Actually, no, it's not a mystical balance, it's that diamond is held together by bonds that are hundreds of kJ/mol; and the mechanical strength of proteins is determined by forces a hundred times as weak as that, the part where proteins fold up like spaghetti held together by static cling."
There is then a deeper story that's even harder to explain, about why evolution doesn't build freely rotating wheels or diamond chainmail; why evolutionary design doesn't find the physically possible stronger systems. But first you need to give people a mechanical intuition for why, in a very rough intuitive sense, it is physically possible to have stuff that moves and lives and self-repairs but is strong like diamond instead of flesh, without this violating a mystical balance where the price of vitalic animation is lower material strength.
And that mechanical intuition is: Deep down is a bunch of stuff that, if you could see videos of it, would look more like tiny machines than like magic, though they would not look like familiar machines (very few freely rotating wheels). Then why aren't these machines strong like human machines of steel are strong? Because iron atoms are stronger than carbon atoms? Actually no, diamond is made of carbon and that's still quite strong. The reason is that these tiny systems of machinery are held together (at the weakest joints, not the strongest joints!) by static cling.
And then the deeper question: Why does evolution build that way? And the deeper answer: Because everything evolution builds is arrived at as an error, a mutation, from something else that it builds. Very tight bonds fold up along very deterministic pathways. So (in the average case, not every case) the neighborhood of functionally similar designs is densely connected along shallow energy gradients and sparsely connected along deep energy gradients. Intelligence can leap long distances through that design space using coordinated changes, but evolutionary exploration usually cannot.
And I do try to explain that too. But it is legitimately more abstract and harder to understand. So I lead with the idea that proteins are held together by static cling. This is, I think, validly the first fact you lead with if the audience does not already know it, and just has no clue why anyone could possibly possibly think that there might even be machinery that does what bacterial machinery does but better. The typical audience is not starting out with the intuition that one would naively think that of course you could put together stronger molecular machinery, given the physics of stronger bonds, and then we debate whether (as I believe) the naive intuition is actually just valid and correct; they don't understand what the naive intuition is about, and that's the first thing to convey.
If somebody then says, "How can you be so ignorant of chemistry? Some atoms in protein are held together by covalent bonds, not by static cling! There's even eg sulfur bonds whereby some parts of the folded-spaghetti systems end up glued together with real glue!" then this does not validly address the original point because: the underlying point about why flesh is more easily cleaved than diamond, is about the weakest points of flesh rather than the strongest points in flesh, because that's what determines the mechanical strength of the larger system.
I think there is an important way of looking at questions like these where, at the final end, you ask yourself, "Okay, but does my argument prove that flesh is in fact as strong as diamond? Why isn't flesh as strong as diamond, then, if I've refuted the original argument for why it isn't?" and this is the question that leads you to realize that some local strong covalent bonds don't matter to the argument if those bonds aren't the parts that break under load.
My main moral qualm about using the Argument From Folded Spaghetti Held Together By Static Cling as an intuition pump is that the local ionic bonds in bone are legitimately as strong per-bond as the C-C bonds in diamond, and the reason that bone is weaker than diamond is (iiuc) actually more about irregularity, fault lines, and resistance to local deformation than about kJ/mol of the underlying bonds. If somebody says "Okay, fine, you've validly explained why flesh is weaker than diamond, but why is bone weaker than diamond?" I have to reply "Valid, iiuc that's legit more about irregularity and fault lines and interlaced weaker superstructure and local deformation resistance of the bonds, rather than the raw potential energy deltas of the load-bearing welds."
Okay. I'm going to take you at your word that you understand that biology is, at it's core, almost entirely built out of covalent bonds. In which case, I am utterly flabbergasted at the way you chose to communicate here.
I think the folded spaghetti gives the wrong impression (spaghetti is not hard to break apart). Let's instead talk about a structure which has at it's core a large steel wire (representing covalent bonds), where parallel sections are glued to each with extremely strong glue(that is obviously weaker than steel bonds) to build a backbone, and then finally those backbones are folded into a weird shape, and joined together at various points with a combination of steel welds, superglue, and sometimes bits of string (representing Van der waals forces). We can call this chunk a "glorpein".
Now I come around, and I want to point out the problems with glorpein. I then proceed to say statements like:
"Glorpeins are held together by string instead of steel wires!"
"Glorpeins are held together by string, which is much weaker than steel wires! "
"My design has figured out how to use steel, instead of Glorpein, which sticks to string! "
"Perhaps, one day in the future, can we build steel equivalents to Glorpein"
I think it's perfectly reasonable to point out that Glorpein is made out of fucking steel. And if you actually know the structure of Glorpein, then these statements are lies, accidental or not, designed to exaggerate the weaknesses involved.
Of course I know that diamond is stronger than bone, and why that is. My job is to simulate crystals! This point was already included in my article:
My point is that by reducing biology down to "static cling", you greatly exaggerate it's weakness, and the comparative advantage of non-biology. As just one example, you can give a protein a blast of heat that breaks all the non covalent bonds... and then often once the heat leaves, it will reform itself right back to what it was before, because it's still held together by covalent bonds. This is one reason why you can't just reduce things to their weakest links, and forget about the other 99% of what holds it together.
And again, while diamond is stronger than proteins, it's also a lot stiffer, less flexible, and less versatile, which is why attempts to build diamond based nanomachines have so far failed. Enzymes can do impressive things because they are flexible and squishy, not in spite of it.
In conclusion, while I understand that science communication is hard, it's not an excuse for saying things that are factually incorrect.
I'm sort of skeptical that you could write something that works as science communication for a general audience, though lord knows I'm not necessarily succeeding either. The key valid ideas to be communicated are:
Now, instead of talking about human cell membranes being held together by static cling, I could talk about extremely thin metallic twisty-tie wires with some magnetized sections that help them fold up together into particular configurations in a barrel of magnetized ball bearings. Your suggestion above for science communication is that this is a great thing to mention, because it helps convey the following interesting truth: if we churn the ball bearings hard enough to unfold the twisty tie, it'll sometimes fold right back up into the same shape again once we stop churning!
This more complicated metaphor may legit add something to an explanation of organic chemistry. I don't disagree that it's cool, or important to organic chemistry proper.
From the perspective of explaining how you die when you confront an uncaring mind that thinks smarter and much faster than humanity, it doesn't add anything not already contained in "cell membranes are held together by static cling".
To be clear, my main objection is that you have made statements that are implicitly or explicitly false. I go over each one in detail in the comment here. Yes, simplification is inevitable, but at many points you crossed the line into saying things that are flat out untrue.
I am confused by the pushback and downvotes in response to pointing this out. Do you not want to be making the strongest argument you can here?
I don't think it's particularly hard to explain why drexlerian nanotech, if it worked, would be powerful and dangerous, without making any implicitly or explicitly false claims.
"Biology is structurally limited by what can be produced by the DNA/RNA system. For example, proteins are built by stitching together a long chain of molecules which fold into themselves to form 3d structures. The backbone is made of strong covalent bonds, but the full 3d structure has weak links where the backbone is pinned together by a variety of forces, some of which are quite weak. In contrast, Drexler style nanotech could be made factory style, layer by layer, and build densely bonded crystalline structures like diamond that are strictly covalently bonded and contain no weak links, and could therefore survive in much hardier conditions and slice through regular cells."
Too long? Okay, here's a quick two sentence version:
"Proteins are made of long chains that fold together and are pinned in place by a variety of forces, some of which are weak. In contrast Drexlerian nanotech could be made out of densely bonded crystalline structures with strictly covalent bonds and no weak links"
If you want to use these arguments, I expect payment in social capital.
Of course, my crux here would be that I don't think Drexlerian nanotech would actually practically work, (part of the reason being the lack of flexibility), but that's a debate for another day.
I don't think this is a fair comparison. If nature wanted skin to be harder, it can do that, for instance with scales (particularly hard in the case of turtle shells). Of course your logic explains why diamond is harder than bone. But if you want a small thing that could penetrate flesh, we already have it in the form of parasites.
It's not clear to me that covalent bonds aren't the ones that are breaking under load when talking about flesh though.
Covalent crosslinks (such as the disulfide bond you mentioned earlier) aren't merely an irrelevant edge case, proteins like collagen (which is used in the extracellular matrix and connective tissue) and keratin (used in hair, nails, horns and hooves) also have such crosslinks.
Why is horn weaker than diamond?
A short and oversimplified answer is that the keratin in horn is not as densely linked with bonds as diamond is, and consequently the atoms are less confined (in a way diamond is sort of like a maximally crosslinked material, though it's not usually described that way).
Generally speaking, crosslinking polymers (including proteins) tends to increase their rigidity. To use a non-living example, when latex is treated with sulfur, the polymer chains also get crosslinked with those same disulfide bonds, producing "vulcanized" rubber which is harder and tougher.
The crosslinks are why you'll sometimes see people say that vulcanized rubber is "one big molecule" (though in practice it's hard to tell if the crosslinking was actually so complete and to link every polymer chain). This is also why vulcanized rubber doesn't really melt, increasing the temperature will cause chemical changes instead (and while I'm not sure, my educated guess it that something similar would happen if you try to melt animal horns).
P.S. I didn't bring it up earlier, but I don't think your earlier statement about the way the masses of the atoms affect the bond strength is accurate. As a counterexample I'd point out that the deuterium-oxygen bond in heavy water is actually a little stronger than that of the protium-oxygen bond in regular water, and in that case the only change is using a more massive form of hydrogen.
What I'm pointing at there is that for strength/weight purposes, using big calcium nuclei to create stronger individual bonds in bone, is like making a steel beam stronger by putting more steel into it; the strength costs weight.
As a physicist, I think your understanding of bonds is a little off here.
Using bigger nuclei usually makes an atom bond weaker, rather than stronger, for reasons to do with the quantum mechanical bond natures. See this explanation for why si-si bonds are weaker than C-C bonds. The simplest explanation for why is simply that the bonding electrons are much further out in heavier elements, because more atomic shells have been filled, so there is correspondingly less force of attraction between them.
I was a little confused learning this initially because i thought that the extra protons in the nuclei would have a bigger attractive effect, but then I remembered that the extra protons come along with extra electrons, so overall the effect is much more complicated and averages out to bigger atom= weaker bond.
But as @Thomas Pilgrim and the linked post pointed out, there are exceptions to this rule due to the intricacies of particular types of bonds, and you really have to dig into the quantum mechanical nature of things to be sure.
Why is crystalline silicon weaker than diamond?
They have the same type of bonds, and the exact same structure. Diamond is harder because not every type covalent bond is equally strong (as you already noted when discussing bone).
Diamond is (close to) the hardest material in the world, because C-C bonds are quite strong, and each carbon atom has four of them. Diamond has C-C bonds densely packed in every direction.
I don't know as much about keratin specifically. This source says some keratin-associated proteins have as much as 41% of their structure consisting of cystine (the amino acid with sulfur attached), so presumably it is also densely packed with Di-Sulfide covalent bonds.
It also says:
So there you go, a clearcut case of protein being held together with covalent bonds. I mean, I still think "the primary structure is 100% covalently bonded" is sufficient to say this, but whatever.
Why is this not stronger than diamond? Well, i would guess that while the dominant bonds are covalent, they are weaker covalent bonds than C-C bonds, and there are not as many of them per atom as in diamond.
Also, we've been talking a lot about hardness here, but it's not the only measure of "strength" you can use. If I cherry-picked fracture toughness, I could say that diamond is weaker than wood, because the fracture toughness of wood is higher than diamond. Check out this video of a diamond being shattered with a regular hammer! Being able to deform and then rebound back into place offers advantages in many situations, and it's why wood and metal doesn't similarly shatter.
To be clear, I obviously still think diamond is stronger than wood along most other measures, such as melting temp, hardness, etc. But there is not zero cost to the rigidity and stiffness of diamond.
This is a relatively minor (but interesting!) point though, please do not only respond to the last two paragraphs.
Seems like weird phrasing but ultimately true. Bones give structure but they're not sticky - if you took away all the flesh (muscle, skin, ligaments etc.) the bones would just fall apart, but if you took away all the bones it would be very floppy but still would clump together. A boneless chicken thigh doesn't fall apart, but a skeleton from hundreds of years ago, where only the bones remain, is not held together.
I have a degree in chemistry and I can fact-check this post as true and that Eliezer is, at best, drawing up bad analogies and most likely just completely misunderstands chemical bonding in biology.
I also think most people with a high school knowledge of chemistry and biology would have been able to figure this out with a bit of thinking; I'd like to think I would have if I had known Eliezer had been writing this. I thus am a bit disappointed that this wasn't pointed out sooner.
Here is an explanation of my problems with each of Yudkowskys claims. I thought this was fairly obvious from the post, but perhaps it needed more explaining. I will go from most to least wrong.
This is just straight up, explicitly false. Biology does not "stick to ionic bonds and proteins". As I pointed out, biology is made up of covalent bonds at it's very core, and uses them all the time.
The phrase "covalently bonded equivalents to biology" implicitly states that biology is not covalently bonded. This is false.
The context of this claim is that Yudkowsky is trying to come up with a new name for deadly Drexler-style nanomachines. He has chosen "covalently bonded bacteria", implying that "covalently bonded bacteria" and normal bacteria are different things. Except that's not true, because bacteria is completely full of covalent bonds.
Okay, I just saw this one, but ribosomes are not "general" assemblers, and they cannot replicate "most other products of biology". They do literally one thing, and that is read instructions and link together amino acids to form proteins.
For the next two. let's establish the principle that if you say "X is held together by Y instead of Z", you are implicitly making the statement that "X is not held together by Z", or perhaps that "Z is irrevelant compared to Y when talking about how X is held together". Otherwise you would not have used the word instead of. Would you utter the phrase "animal bodies are held together by flesh instead of skeletons?"
This implicitly makes the statement "proteins are not held together by strong covalent bonds", which is false. Or it could be saying "strong covalent bonds are irrelevant compared to van der waals forces when talking about how proteins are held together", which is also false.
even though the proteins are held together by van der Waals forces rather than covalent bonds, which is why algae are far less tough than diamond
"rather than" means the same thing as "instead of", and therefore makes an implicitly false statement for the reason I said in the last quote.
Actually, this one is defensible, because it didn't use the phrase "instead of". I would still prefer more qualifying terms such "the weakest link". If this had been the only statement, I would not have written this post.
I agree that "biology sticks to ionic bonds and static cling" was badly put because lignin, and I'll retire that one.
The average physics textbook contains multiple errors of the order, “forgot to include the normalization term.”[1] To me, it seems incorrect to bring up Eliezer's error as strong evidence that he doesn't know what he's talking about, without acknowledging the base rate of similar errors.
(I recognize that you also point to Eliezer's economics errors. I haven't read Inadequate Equilibria or the post critiquing it, so I can't comment on the magnitude of the errors there, but I think a base rate assessment—e.g., how frequently do academic-level economics books turn out to have a shaky thesis?—should again apply.)
Based on my experience studying the subject at university, at least.