Summary
- Life history classification will hide some significant differences in the lives of wild animals. Not all species within a given classification possess all of the traits associated with that group even across all years or all locations. Therefore, when making moral decisions, one also has to consider how average quality of life should be determined in the face of large variance
- Among insect herbivores, some lifespans are relatively long, some modes of death are very quick, and some small-bodied herbivores may lead lives characterized by ample food resources
- Although determining the affective states of wild animals from this data is impossible, it seems quite likely that the majority individuals in some subgroups, such as those sheltered from both the elements and predation by feeding from within plant tissues, lead very high quality lives
- Knowing a group of organisms produce many offspring, have high mortality rates, small body size and are short-lived is not sufficient to determine that their lives are a net negative (or positive)
The argument from life history suggests that since many species produce many more offspring than survive to adulthood, are of small size and so subject to many abiotic and biotic threats, and are short-lived relative to humans, that there is more suffering than happiness in nature and therefore we have a moral obligation to end this suffering (e.g., Tomasik 2015). Here, we do not attempt to examine such moral quandaries (for a thought experiment on these issues see Brennan 2017). Instead, we aim to improve the quality of discussions by examining available data on one group of wild animals. Because of their broad scope, some previous analyses of wild animal welfare have lumped many species together at one pole of a continuum of life history strategies. Given these large groupings, and some issues with the life history classifications themselves, it is unclear to what extent this approach actually informs us about animal suffering in general. On the other hand, examinations of the lives of particular species are also unsatisfactory, since they refer to the specific rather than the majority. By narrowing our focus to one group, we may be able to bring more data to bear on our intuitions regarding wild animal welfare. In this post, we explore the literature regarding one group of organisms that are classically grouped in the “r-strategist” life history category: terrestrial insect herbivores.
Terrestrial insects are described by some as having net negative welfare. They are one of the largest groups of terrestrial animals in terms of number of species, biomass and estimated number of individuals. As May (1988) noted, “To a rough approximation, and setting aside vertebrate chauvinism, it can be said that essentially all organisms are insects.” Bar-On et al. (2018) estimate the biomass of terrestrial arthropods at 0.2 gigatons of carbon, and insects are the largest classification in the arthropod phylum, accounting for over 80% of all described species in this group (Zhang 2013). For example, Hölldobler & Wilson (1990) suggest that 15-20% of the biomass of terrestrial animals is composed of ants. Insects are ~1/3 of all described species on the earth (Grimaldi & Engel 2005), while estimates for the total number of insect species range between five and seven million (e.g., Stork 2018).
Various approximations of the total number of insect individuals range between 10^17-10^19 (Williams 1960 , Hölldobler & Wilson (1990), Bar-On et al. (2018)). As small-bodied primary consumers, herbivores will be at the bottom of the trophic energy transfer in any community. Since only ~10% of primary production passes between trophic levels, this group and detritivores will make up ~90% of all terrestrial insects. Assuming equal division between these two groups and 1018 insect individuals, terrestrial insect herbivores will then comprise approximately 10^17 individuals. In other words, this is one of the largest groups of terrestrial animals on the earth.
Life history classification of terrestrial insects
Terrestrial insects are often grouped near the “r” or “fast” end of life history classifications (see our prior post on life history). The r- and K-selected classification scheme is based on the different evolutionary pressures in uncrowded, resource rich environments and crowded resource poor environments. (MacArthur and Wilson 1967). K-selected species will frequently experience resource scarcity and starvation, while r-selected species will experience an abundant environment. Extreme r-strategies are small-bodied and short-lived, reach reproductive maturity early, reproduce once (semelparous), have a large number of offspring at that time, do not provide parental care, and have high juvenile mortality (Pianka 1970). The fast-slow categorization of life history that has superseded r- and K- classification has similar traits for “fast” species. However, there will always be exceptions: eggplant lace bugs provide extensive parental care (Tallamy 1999), and cicadas can live for nearly 2 decades (Simon 1988). Moreover, some species have life histories that are not captured by these dichotomous classifications. In particular, those in harsh abiotic conditions may have longer lifespans, slower growth rates, and lower reproductive output, because of a larger investment in survival adaptations (e.g., arctic wooly bears Kukal & Dawson 1989).
Analyses of terrestrial insect herbivore life history
Here we report on available literature data regarding fecundity, mortality and lifespan in an effort to provide some quantitative insight regarding the lives of terrestrial insect herbivores. We conclude that variability in life history is the primary characteristic of this group, but certainly these species are, on average, shorter-lived, more fecund, and less likely to provide parental care than mammals.
Life span
Both r-selected and fast life history classifications describe insects as short-lived, and most do live less than two years. Life spans of insects, however, are quite variable. Carey (2001) notes that the between-group variation is enormous: for herbivores, this range includes aphids with a lifespan of weeks, to xylem-feeding beetles that take several years to reach maturity, to termite queens that can live for decades. This 5000-fold difference in the life spans of insects can be contrasted with the smaller 40-fold difference in the life spans of mammals: from small rodents that live less than 2 years to humans that live an average ~80 years.
It is incorrect to believe that life span for any insect species is a single fixed age. The lifespan of a monarch butterfly (Danaus plexippus) is approximately 2-3 months when it is in the reproductive mode during the summer months but 6-10 months when it is in the migratory mode during the winter months (Grace 1997). Some commentators have confused the length of a single life stage with that of the entire lifespan of an individual. For example, while some mayfly adults live only a few hours, mayfly nymphs take longer, up to two years, to mature. For some species (e.g., Lepidoptera: butterflies and moths), these juvenile stages tend to be the largest proportion of the total lifespan in temperate regions (e.g., see data in Danks 2006 for adult life spans). For others, (e.g., Coleoptera: beetles), the adult life stage tends to be the longest. For species without distinct larval and pupal stages, the prereproductive stage also tends to be shorter than the adult stage (e.g., Hemiptera: true bugs). We note that these are all very coarse generalizations over very large groups. There are about 177,500 described species of Lepidoptera, 400,000 of Coleoptera, and 79,000 of Hemiptera.
Across insect groups, parental care, monogamy, and eusociality (at least for queens) are all associated with extended life spans. As well, species that must seek out host plants that are scarce or widely dispersed tend to be long-lived (e.g., Heliconius butterflies have widely-dispersed host plants and only lay only a few eggs at a time. These species are long-lived for lepidopterans in temperate locations, sometimes exceeding 4–6 months (Ehrlich 1987, Gilbert 1972).
As ectotherms, total lifespan and development time are also related to temperature for insects. For example, at 10 °C Aphis gossypii take 75 days to reach reproductive maturity, and live a maximum of 103 days, while at 30 °C they take 5 days to reach reproductive maturity and live a maximum of 37 days (Kocourek et al 1994). In addition, increased longevity can be associated with periods of resource limitation. Extreme life extensions, such as diapause for more than 10 years, usually affect only a very small fraction of the population, and have only been recorded in about 64 species (Gill et al. 2017). However, more modest extensions, such as development over 2 years when 1 year is more normal, are relatively common (Danks 1992).
In addition, species that are subject to uncertain or harsh environments frequently exhibit extended longevity associated with extended or repeated diapause. Overwintering diapause normally lasts for 9-10 months in the temperate zones (Gill et al. 2017). However, Convey (1997) reports extended lifespan in Antarctic arthropods as compared to their close phylogenetic relatives in more temperate regions (e.g., 2-5 years vs ~1 year) associated with repeated diapause over several winters. However, lifespan can be shortened or lengthened to deal with a brief summer season in polar regions (Danks 2004). Given this, we generally expect increased variability in lifespan with increasing latitude and also altitude (e.g., Laiolo & Obeso 2017).
Overall, very short lifespans (less than 20 days) seem fairly rare (Danks 2006), and long lives (>3 years) are rarer still. Species from cool temperate regions tend to have longer life cycles with about one generation per year (e.g., Danks and Foottit 1989), as do species living in areas that have a dry season. But we note that for many of these species, variable environmental conditions determine how many generations there are per year, and in addition, the overwintering generation will have a longer lifespan than growing season generations.
Fecundity
The number of offspring produced by terrestrial herbivores varies with body size, parental care, and the environment. While infant care is not common among insect species, it certainly occurs, and is negatively correlated with reproductive output as expected from a fast-slow life history classification. Gilbert and Manica (2010a) examined fecundity data for 220 non-caring, 23 offspring guarding, and 32 offspring provisioning terrestrial insects (Note: this analysis was not restricted to herbivores, but did exclude eusocial species). Fecundity is defined as the number of offspring produced over the lifetime of an individual. Dung beetles species had the lowest lifetime fecundity (~2 offspring), while mayflies had the largest (~4000 offspring). For terrestrial herbivores in this dataset, the range extends from 12-15 offspring for some leafminers and wood-boring beetles to ~3000 for cutworms. The median lifetime fecundity (measured as number of eggs per individual) was 138 but varied with the type of parental care (166 eggs for no care, 66 for guarding and 40 for provisioning insects; all values calculated from Gilbert and Manica 2010b; but note the authors did not identify the number of eggs that were actually viable).
Contrary to the fast-slow life history classification, fecundity often, although not always, positively correlates with body size in insects (see Leather 1988 for discussion). In species where parents provide no care or simply guard eggs (e.g., the hibiscus harlequin bug Tectocoris diophthalmus aggressively defends newly laid eggs, Giffney & Kemp 2016), larger-bodied species produced more and larger eggs. In species that provision offspring, such as burrower bugs that provide mint nutlets to their nymphs (Sehirus cinctus, see Kight 1997), those with larger bodies also produced larger eggs but laid fewer (Gilbert and Manica 2010a). In this respect, the life histories of provisioning insects resembled those of birds or mammals (traditionally viewed as slow or K-selected species) rather than those of related species that invested less in parental care.
In addition to the form of parental care and body size, environment also has an impact on insect fecundity. Herbivores that feed on the plant exterior have higher fecundities (Cornell & Hawkins 1995), but this finding is partially confounded by the fact the external feeders tend to have larger body sizes than species that feed from within plant tissues (e.g., endophytics such as gall formers, stem borers and leaf miners). More generally, plant quality can alter the fecundity of insect herbivores (Awmack & Leather 2002).
Temperature also determines fecundity, for example mean total fecundity of Aphis gossypii ranges from 36 larvae per female at 10 °C to 76 larvae at 30 °C. (Kocourek et al. 1994). Broader environmental conditions also impact fecundity through both effects on time to reproductive maturity and egg viability. For example, the developing larvae of the arctic woolly bear moth, Gynaephora groenlandica, feed on a northern willow species only in June when ambient temperatures are relatively high and the host plant has the highest nutrient content. The larvae then leave the plant until the following summer. Because of the short growing season, the moth larvae take between 7-14 years to reach maturity (Kukal & Dawson 1989, Morewood and Ring 1998), and females have low lifetime fecundity, producing ~ 5 viable eggs (Kukal & Kevan 1987).
Juvenile Mortality
Survival trajectories for juvenile insect herbivores are quite variable, but do show a trend of higher mortality for younger stages. The most frequent cause of mortality is predators and parasitoids. Cornell & Hawkins (1995) examined 530 datasets for 124 species of herbivorous insects that had 4 distinct stages: egg, larvae, pupae and adult regarding the survival of the juvenile stages to determine the most frequent causes of death. Sprayed, caged, or laboratory populations were excluded from analyses, as were insects that had no distinct pupal stage (i.e., these are species such as beetles and butterflies, but not aphids). The most common cause of death across both groups is predators and parasitoids followed by weather, plant defences and competition (Fig 1).
However, the authors find wide variation in survival trajectories regardless of whether the same species and population was sampled within the same year in different locations or between years. Between species, this variance is even larger. For example, in most cases 50% of eggs of Dacus oleae, the olive fruit fly, survived to adulthood, while less than 5% of cabbage root fly (Erioschia brassicae) eggs did. If an average across this wide variance is taken, there are similar survival trajectories of juveniles across species groups, with slightly higher rates of mortality occurring in the early life stages and slightly lower rates in the later ones. Sources of mortality shift as herbivores grow. Physiological factors, weather and plant factors more frequently kill early stages, whereas parasitoids and predators more frequently kill later stages.
External feeders were more likely to be killed by parasitoids and predators, while plant factors were more likely to kill species that feed from inside the plant tissue (endophytic species). This group of herbivores had higher survival in the older juvenile stages than species that fed from the outside of the plant (Cornell & Hawkins 1995). On average about 70% of eggs became larvae, 20-30% of eggs became late stage larvae (depending on whether the species was endophytic or not), 5-20% became pupae and 2-10% of eggs reached adult stage. These findings broadly support Price's (1974) earlier claim of lower mortality in larvae concealed in plant tissue, but not the claim that this group exhibited convex survival curves, with most mortality occurring past the mid- to late larval stage.
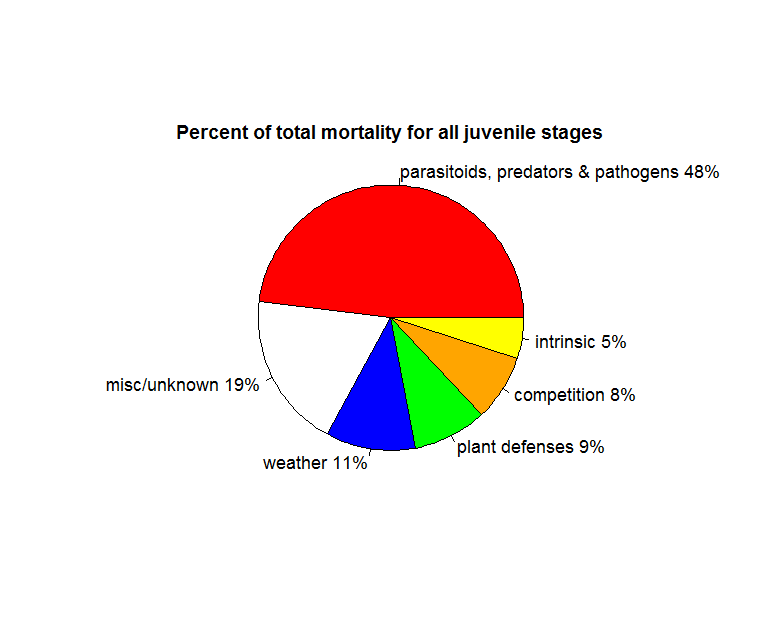
Figure 1: Summary of the frequency of mortality factors for all juvenile insect herbivores in Cornell & Hawkins (1995).
Competition
The initial idea behind r-selection, that species on this end of the spectrum are not limited by resources may be supported by Cornell and Hawkins’s (1995) analysis of mortality. Competition is a very small factor in total juvenile mortality, and the vast majority of mortality events are caused by predators and parasitoids. The weather, intrinsic factors related to reproduction (egg viability) and plant defences also explained more mortality than competition. This lower impact of competition is possibly because there are very few species that reach very large population sizes (e.g, Ayres and Lombardero 2000, Faeth 1987). In addition, even among these species, such population outbreaks are rather rare, usually local and of short duration (Kozlov & Zvera 2017).
On the other hand, meta-analyses consistently find evidence of negative effects of one insect herbivore species on another (Kaplan & Denno 2007, Bird et al. 2019); however, the suggested mechanisms for this impact are predominantly indirect effects (e.g., inducing stronger plant defences and increasing predator populations). Direct lethal impacts caused by food shortages are considered less common. Moreover, in a meta-analysis of 75 published studies Vidal & Murphy (2018) found that predators and parasitoids have a larger impact on herbivore abundance, survival, growth and fecundity than plant quality. There are also significant beneficial effects of insect herbivores species on each other (e.g., by overwhelming host plant defences, distracting natural enemies, or by creating shelters such as leaf mines and rolls; Cornelissen et al. 2016, Soler et al. 2012), so that there is no necessary direct relationship between increasing numbers of herbivores and negative impacts.
Predation, pathogens and parasitoids
Natural enemies (parasitoids, predators and pathogens) emerged as the most frequent mortality factor (48% of all deaths) in Cornell and Hawkins (1995), surpassing every other source of mortality over all life stages and, in some cases, exceeding all others combined. These factors were a larger source of mortality in later juvenile stages. In a study of 78 insect herbivore species with a range of 100-1000 individuals for each, Hawkins et al. (1997) report close to 0% median percent mortality for eggs and early larval stages from natural enemies, although this statistic is partially due to the extremely low mortality among endophytic species. In this study, natural enemies killed a median of 1% of mid larval stage individuals, 3% of late stage and 5% of pupal stage.
Hawkins et al. (1997) find that overall ~4% of all individuals are killed by parasitoids, ~1% by predators and less than 0.5% by pathogens. These authors report that many herbivores, particularly species feeding within plant tissues, suffer little or no mortality from pathogens. So while the authors note that disease can be important in some groups of insects (e.g., forest Lepidoptera Myers 1993), on average it does not seem represent an important mortality source in phytophagous insect populations. Hawkins et al. (1997) also find that parasitoid deaths are more common in temperate regions than in tropical regions, where predators have a somewhat larger impact. In addition, both Cornell and Hawkins (1995) and Hawkins et al. (1997) report that endophytic herbivores suffer lower mortality by predators. Leaf miners suffer the greatest parasitoid-induced mortality, while gall-formers, stemborers and root feeders suffer the least. Hawkins et al. (1997) note that for koinobiont parasitoids (see below), attack will occur in earlier larval stages, while death will occur later. So herbivore mortality rates increase through time, where this mortality includes both both delayed mortality from koinobionts as well as immediate mortality by later attacks from predators and idiobiont parasitoids.
Predators
True predators are less specialized than parasites and parasitoids, and they usually catch prey smaller than themselves (Griffiths 1980). It has been suggested that the impact of death by predation on the net value of a life may be partially determined on the length of the predation event with respect to length of life. Since insect life spans are extremely variable, so are the length of juvenile stages. However, as noted by Plant (2016), death by predation is often a relatively small proportion of even a very short life span. For example, video capture of a generalist predator Harmonia axyridis (Asian multi-coloured ladybug) feeding on aphids suggests consumption rates of 20-60 aphids in a one hour period, implying that each prey individual was consumed in a couple of minutes (Feng et al. 2019). In contrast Michálek et al. (2017) used high speed photography to capture hunting tactics in both a specialist and generalist spider species. Specialist predators tend to consume relatively large prey. The authors recorded body part consumption rates, and if we assume that prey death occurred after maximum consumption time by the specialist predator, then the prey would experience about 300 minutes of predation. Using these times, and noting that the majority of juvenile mortality by predation occurs in the late larval to pupal stage, which for the shorter lived species would be on the order of a couple of weeks, the experience of death by a true predator would range from 0.007-1.0% of total lifespan (assuming death at 3 weeks). For species that tended to take longer to mature (e.g., ~1 yr), and assuming that the late larval or pupal stage occurs occurs in the fall, the average estimate would be lower (~0.002-0.3% of total lifespan, assuming death at 3 months).
Parasitoids
Across all stages, parasitoids kill more herbivores than either predators or pathogen (Hawkins et al. 1997). Death by parasitoid is most common late larval and pupal stages (Hawkins et al. 1997). Koinobiont parasitoids (cf Harvey & Malcicka 2016), such as Cotesia vestalis, allow the host to continue feeding and develop so that death takes quite a long time. In contrast idiobionts cause host development to cease once parasitized, either by causing death or paralysis during oviposition. Therefore, juvenile herbivores inoculated by parasitoids can experience a much longer time frame to death (e.g., 12 days in one study). However, this species group is enormous, and it seems difficult to make any generalizations regarding time to death by different classes of parasitoids. Koinobiont parasitoids often represent the most speciose portion of parasitoid complexes (Mills 1994), so it is possible that this is the most common form of death for insect herbivores.
The percentage of time the insect herbivore experiences the effects of the parasitoid will be highly variable, but related to the age at which is parasitized. If we assume inoculation at the most common time of early-mid larval stage, and a koinobiont parasitoid that keeps the larvae alive for another 10 days of development, then ~40% of total lifespan may serve as an estimate for species that mature in about 60 days (death at just over 3 weeks), and ~20% for those that take about 1 year, where late larval or pupation stage occurs at about 4 months (i.e., September - October in northern temperate regions, and therefore death at ~6 weeks). However, whether larvae being eaten by parasitoids feel pain during the experience is unclear, given that both parasitoids and parasites can cause extensive behaviour modification in their hosts (e.g., Chen et al 2017).
Weather
Following unknown or miscellaneous factors, the weather was the second largest known cause of mortality (11%) in Cornell and Hawkins (1995), and this factor had a larger impact on early juvenile states. Weather-generated mortality was due mainly to rainfall and overwintering deaths. The higher frequency of weather-generated mortality in early larval stages is probably due to higher risk of dislodgement by rainfall. The authors suggest that the importance of weather in later stages probably depends on whether these stages overwinter. Of course, species that live within the plant tissues (e.g., gall-formers, stem borers and and leaf-tiers) were less affected by weather than species that live on plant surfaces. Gregarious species are also less likely to be affected by weather conditions. For example, tent caterpillars raise their temperatures by basking in groups and also construct elaborate group shelters (Stamp and Bowers 1990). We also point out that even extremely harsh condition are not necessarily a problem for species adapted to those locations. Arctic woolly bear winter mortality is quite low (~13%) because the larvae seek out well protected locations and have special physiological adaptations for cold hardiness (Kukal et al. 1987, 1989).
Therefore, mortality of juvenile herbivores can be quite high, but then again, the juvenile stage may be the longest life stage for some of these species (e.g., Lepidopterans). Mortality of juvenile herbivorous insects is dominated by parasitoids and predators, in that order, in temperate regions. Endophytic species are less likely to die from these causes (with the exception of leaf miners). Juvenile insect herbivores seem to be seldom short of food (although food quality may vary), and are only occasionally significantly impacted by weather.
Conclusion
This examination of data on insect herbivores suggests that life history classification will hide some significant differences in the lives of wild animals. In particular, claims about large fecundity, lack of parental care and short lives are not correct for all species that are represented as belonging to a single group within a life history classification, nor are they true for all years or all locations of the same species. Therefore, when making moral decisions about the quality of wild animal lives, it seems likely that one also has to consider one’s position regarding how average quality of life should be determined in the face of such large variance.
Setting aside problems with generalization, we note that the mere observation a large group of organisms produce many offspring, have high mortality rates, small body size and are short-lived is still not sufficient to determine that their lives are net-negative. Even if death is very painful, it is unclear that experiences previous to death are not sufficiently pleasurable to compensate. While this possibility has often been dismissed on the grounds that these animals have such a short lifespan, some of the data we explore suggest that some juvenile lifespans are relatively long, some modes of death are very quick, and that small-bodied herbivores may often lead lives characterized by ample food resources. In fact, it seems quite possible that the majority individuals in some subgroups, such as those sheltered from both the elements and predation by feeding from within plant tissues, lead very high quality lives. We are careful to note, however, that attempting to determine the affective states of wild animals from this data is currently impossible.
References
Awmack, C. S., & Leather, S. R. (2002). Host plant quality and fecundity in herbivorous insects. Annual review of entomology, 47(1), 817-844. https://doi.org/10.1146/annurev.ento.47.091201.145300
Ayres, M. P., & Lombardero, M. J. (2000). Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Science of the Total Environment, 262(3), 263-286. https://doi.org/10.1016/S0048-9697(00)00528-3
Bar-On, Y. M., Phillips, R., & Milo, R. (2018). The biomass distribution on Earth. Proceedings of the National Academy of Sciences, 115(25), 6506-6511. https://doi.org/10.1073/pnas.1711842115
Bird, G., Kaczvinsky, C., Wilson, A. E., & Hardy, N. B. (2019). When do herbivorous insects compete? A phylogenetic meta‐analysis. Ecology Letters. https://doi.org/10.1111/ele.13245
Brennan, O. 2017. Infant mortality and the argument from life history. Retrieved 02.05.19 https://was-research.org/blog/infant-mortality-argument-life-history/
Carey, J. R. (2001). Insect biodemography. Annual Review of Entomology, 46(1), 79-110. https://doi.org/10.1146/annurev.ento.46.1.79
Chen, W. B., Vasseur, L., You, M. S., Li, J. Y., Wang, C. X., Meng, R. X., & Gurr, G. M. (2017). Parasitised caterpillars suffer reduced predation: potential implications for intra-guild predation. Scientific reports, 7, 42636. https://doi.org/10.1038/srep42636
Convey, P. (1997). How are the life history strategies of Antarctic terrestrial invertebrates influenced by extreme environmental conditions?. Journal of Thermal Biology, 22(6), 429-440. https://doi.org/10.1016/S0306-4565(97)00062-4
Cornell, H. V., & Hawkins, B. A. (1995). Survival patterns and mortality sources of herbivorous insects: some demographic trends. _The American Naturalis_t, 145(4), 563-593. https://www.journals.uchicago.edu/doi/abs/10.1086/285756
Cornelissen, T., Cintra, F., & Santos, J. C. (2016). Shelter-building insects and their role as ecosystem engineers. Neotropical Entomology, 45(1), 1-12.https://doi.org/10.1007/s13744-015-0348-8
Danks, H. V. (1992). Long life cycles in insects. The Canadian Entomologist, 124(1), 167-187. https://doi.org/10.1007/s13744-015-0348-8
Danks, H. V. (2004). Seasonal adaptations in arctic insects. Integrative and Comparative Biology, 44(2), 85-94. https://doi.org/10.1093/icb/44.2.85
Danks, H. V. (2006). Short life cycles in insects and mites. The Canadian Entomologist, 138(4), 407-463. https://doi.org/10.4039/n06-803
Danks, H. V., & Foottit, R. G. (1989). Insects of the boreal zone of Canada. The Canadian Entomologist, 121(8), 625-690. https://doi.org/10.4039/Ent121625-8
Ehrlich, P .R. (1984). The structure and dynamics of butterfly populations. In R.I. Vane-Wright & P.R. Ackery (Eds.), The Biology of Butterflies (pp. 25-40). London: Academic Press https://books.google.ca/books/about/The_Biology_of_Butterflies.html?id=fP8hAQAAMAAJ&redir_esc=y
Faeth, S. H. (1987). Community structure and folivorous insect outbreaks: the roles of vertical and horizontal interactions. Pgs 135-171. In P. Barbosa & J. Schultz (Eds.), Insect Outbreaks (pp. 135-171). San Diego: Academic Press. https://doi.org/10.1016/C2009-0-02860-8
Feng, Y., Li, Y. D., Liu, Z. G., Yu, X. L., Zhu, G. X., Keller, M., & Liu, T. X. (2019). Behavioural patterns and functional responses of a generalist predator revealed using automated video tracking. Pest Management Science, 75(6), 1517-1526 https://doi.org/10.1002/ps.5314
Giffney, R. A., & Kemp, D. J. (2016). Maternal care behaviour and kin discrimination in the subsocial bug Tectocoris diophthalmus (Hemiptera: Scutelleridae). Austral Entomology, 55(2), 170-176. https://doi.org/10.1111/aen.12164
Gilbert LE. 1972. Pollen feeding and reproductive biology of Heliconius butterflies. Proceedings of the National Academy of Sciences USA 69(6), 1403-1407. https://doi.org/10.1073/pnas.69.6.1403
Gilbert, J. D., & Manica, A. (2010a). Parental care trade-offs and life-history relationships in insects. The American Naturalist, 176(2), 212-226. https://www.journals.uchicago.edu/doi/abs/10.1086/653661
Gilbert J, Manica A (2010b) Data from: Parental care trade-offs and life history relationships in insects. Dryad Digital Repository. https://doi.org/10.5061/dryad.1451
Gill, H. K., Goyal, G., & Chahil, G. (2017). Insect diapause: a review. Journal of Agricultural Science and Technology, 7, 454-473. http://www.davidpublisher.org/Public/uploads/Contribute/5a5c6c5a389c3.pdf
Grace, E.S. (1997). The Nature of Monarch Butterflies. Vancouver, Canada: Greystone Books https://www.amazon.ca/nature-monarch-butterflies-Beauty-flight/dp/1550545701
Greenslade, P. J. (1983). Adversity selection and the habitat templet. The American Naturalist, 122(3), 352-365. https://www.journals.uchicago.edu/doi/abs/10.1086/284140
Griffiths, D. (1980). Foraging costs and relative prey size. The American Naturalist, 116(5), 743-752. https://www.journals.uchicago.edu/doi/abs/10.1086/283666
Grimaldi, D. & Engel, M.S. (2005). Evolution of the Insects. New York: Cambridge University Press https://books.google.ca/books/about/Evolution_of_the_Insects.html?id=Ql6Jl6wKb88C
Grime, J. P. (1977). Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. The American Naturalist, 111(982), 1169-1194. https://www.journals.uchicago.edu/doi/abs/10.1086/283244
Harvey, J. A., & Malcicka, M. (2016). Nutritional integration between insect hosts and koinobiont parasitoids in an evolutionary framework. Entomologia Experimentalis et Applicata, 159(2), 181-188. https://doi.org/10.1111/eea.12426
Hawkins, B. A., Cornell, H. V., & Hochberg, M. E. (1997). Predators, parasitoids, and pathogens as mortality agents in phytophagous insect populations. Ecology, 78(7), 2145-2152. https://esajournals.onlinelibrary.wiley.com/doi/abs/10.1890/0012-9658(1997)078%5B2145:PPAPAM%5D2.0.CO;2
Hölldobler, B., & Wilson, E. O. (1990). The ants. Cambridge, Mass: Harvard University Press. https://books.google.ca/books/about/The_Ants.html?id=o87CQgAACAAJ&redir_esc=y
Kaplan, I., & Denno, R. F. (2007). Interspecific interactions in phytophagous insects revisited: a quantitative assessment of competition theory. Ecology Letters, 10(10), 977-994. https://doi.org/10.1111/j.1461-0248.2007.01093.x
Kight, S. L. (1997). Factors influencing maternal behaviour in a burrower bug, Sehirus cinctus (Heteroptera: Cydnidae). Animal Behaviour, 53(1), 105-112. https://doi.org/10.1006/anbe.1996.0282
Kocourek, F., Havelka, J., Berankova, J., & Jaroŝik, V. (1994). Effect of temperature on development rate and intrinsic rate of increase of Aphis gossypii reared on greenhouse cucumbers. Entomologia Experimentalis et Applicata, 71(1), 59-64. https://doi.org/10.1111/j.1570-7458.1994.tb01769.x
Kozlov, M. V., & Zvereva, E. L. (2017). Background insect herbivory: impacts, patterns and methodology. In: Cánovas F., Lüttge U., & Matyssek R. (Eds) Progress in Botany Vol. 79 (pp. 313-355). Cham: Springer. https://doi.org/10.1007/124_2017_4
Kukal, O., & Kevan, P. G. (1987). The influence of parasitism on the life history of a high arctic insect, Gynaephora groenlandica (Wöcke)(Lepidoptera: Lymantriidae). Canadian Journal of Zoology, 65(1), 156-163. https://doi.org/10.1139/z87-022
Kukal, O., & Dawson, T.E. 1989. Temperature and food quality influence feeding behavior, assimilation efficiency and growth rate of arctic woolly-bear caterpillars. Oecologia 79:526–32 https://doi.org/10.1007/BF00378671
Laiolo, P., & Obeso, J. R. (2017). Life-history responses to the altitudinal gradient. In Catalan, J., Ninot, J. M., & Aniz, M. M. (Eds.). High mountain conservation in a changing world (pp. 253-283). Cham: Springer. https://link.springer.com/chapter/10.1007/978-3-319-55982-7_11
Leather, S. R. (1988). Size, reproductive potential and fecundity in insects: things aren't as simple as they seem. Oikos, 386-389. https://www.jstor.org/stable/3565323
May, R. M. (1988). How many species are there on earth?. Science, 241(4872), 1441-1449. https://science.sciencemag.org/content/241/4872/1441
MacArthur, R. H., & Wilson, E. O. (1967). The theory of island biogeography. Princeton: Princeton University Press. Retrived 23/05/2019 https://books.google.ca/books/about/The_Theory_of_Island_Biogeography.html?id=a10cdkywhVgC
Michálek, O., Petráková, L., & Pekár, S. (2017). Capture efficiency and trophic adaptations of a specialist and generalist predator: a comparison. Ecology and Evolution, 7(8), 2756-2766. https://doi.org/10.1002/ece3.2812
Mills, N. J. (1994). Parasitoid guilds: defining the structure of the parasitoid communities of endopterygote insect hosts. Environmental Entomology, 23(5), 1066-1083. https://doi.org/10.1093/ee/23.5.1066
Morewood, W. D., & Ring, R. A. (1998). Revision of the life history of the High Arctic moth Gynaephora groenlandica (Wocke)(Lepidoptera: Lymantriidae). Canadian Journal of Zoology, 76(7), 1371-1381. https://doi.org/10.1139/z98-085
Myers, J. H. (1993). Population outbreaks in forest Lepidoptera. American Scientist, 81(3), 240-251. https://www.jstor.org/stable/29774919
Pianka, E. R. (1970). On r-and K-selection. The American Naturalist, 104(940), 592-597. https://www.jstor.org/stable/2459020
Plant, M. 2016. The Unproven (And Unprovable) Case For Net Wild Animal Suffering. A Reply To Tomasik Retrieved 02.05.19 https://forum.effectivealtruism.org/posts/guvsD78ZXhfCaT7SH/the-unproven-and-unprovable-case-for-net-wild-animal.
Price, P. W. (1974). Insect ecology New York: Wiley. https://books.google.ca/books/about/Insect_Ecology.html?id=rrzc-IkgNx0C&redir_esc=y
Sæther, B. E. (1987). The influence of body weight on the covariation between reproductive traits in European birds. Oikos, 79-88. https://www.jstor.org/stable/3565691
Simon, C. (1988). Evolution of 13- and 17-year periodical cicadas. Bulletin of the Entomological Society of America, 34, 163–176. https://doi.org/10.1093/besa/34.4.163
Soler, R., Badenes‐Pérez, F. R., Broekgaarden, C., Zheng, S. J., David, A., Boland, W., & Dicke, M. (2012). Plant‐mediated facilitation between a leaf‐feeding and a phloem‐feeding insect in a brassicaceous plant: from insect performance to gene transcription. Functional Ecology, 26(1), 156-166. https://doi.org/10.1111/j.1365-2435.2011.01902.x
Stamp, N. E. and Bowers, M. D. 1990. Variation in food quality and temperature constrain foraging of gregarious caterpillars. Ecology 71, 1031–1039. https://doi.org/10.2307/1937371
Stork, N. E. (2018). How many species of insects and other terrestrial arthropods are there on Earth?. Annual Review of Entomology, 63, 31-45. https://doi.org/10.1146/annurev-ento-020117-043348
Tallamy, D. W. (1999). Child care among the insects. Scientific American, 280(1), 72-77. https://www.jstor.org/stable/26058020
Tomasik, B. (2015). The importance of wild-animal suffering. Relations: Beyond Anthropocentrism, 3, 133. https://doi.org/10.7358/rela-2015-002-toma
Vidal, M. C., & Murphy, S. M. (2018). Bottom‐up vs. top‐down effects on terrestrial insect herbivores: a meta‐analysis. Ecology Letters, 21(1), 138-150. https://doi.org/10.1111/ele.12874
Williams, C. B. (1960). The range and pattern of insect abundance. The American Naturalist, 94(875), 137-151. https://www.journals.uchicago.edu/doi/abs/10.1086/282115
Zhang, Z. Q. (2013). Phylum arthropoda. Zootaxa, 3703(1), 17-26. http://dx.doi.org/10.11646/zootaxa.3703.1.6
Credits

This essay is a project of Rethink Priorities. It was written by Kim Cuddington. Thanks to Jason Schukraft, Daniela Waldhorn, David Moss, Marcus Davis and Peter Hurford for comments. If you like our work, please consider subscribing to our newsletter. You can see all our work to date here.

There's tons of useful info in this piece. :)
I take it that your "Life span" section refers to adult lifespans? For example, the statement that "Overall, very short lifespans (less than 20 days) seem fairly rare" refers to reaching maturity in less than 20 days? Do you have estimates for life expectancy at birth (maybe ignoring egg mortality, assuming eggs aren't sufficiently sentient to warrant concern)? Your sections on "Predators" and "Parasitoids" gave some point estimates based on when predation and inoculation by parasitoids often occur. Maybe those are reasonable approximations for life expectancy at birth. On the other hand, isn't survivorship almost always "concave upward", with most deaths occurring quite early? This figure is one random example, showing that most of the insects are dead before the second instar. And because of the concave-upward shape, the average age of death should be pretty young.
I tend to assume that insects in diapause have relatively little subjective experience, such that those periods of time "don't count" very much if we're using lifespan as a measure of how long the animal experiences pleasure and pain. Of course, if the insect is minimally sentient during that time, then maybe deaths occurring during that time aren't that bad.
Extending this idea, it seems plausible that ectotherms that mature slowly in cool climates have less sentience and less hedonic experience per day than those in warm climates, because biological activity is generally slowed down in cool climates. So maybe the difference in total amount of life experiences is less than one might assume between longer-lived slow-developing insects in high latitudes vs fast-developing insects at low latitudes.
If we imagine only two species of insect -- one with lifetime fecundity of 2 and one with 4000 -- and if each species has equal numbers of egg-laying mothers, then the ratio of (total offspring)/(total mothers) will still be very high: (2 + 4000)/(1 + 1) = 2001. When we make assessments about the net hedonic balance of an entire ecosystem containing multiple species, it's this average value that seems most relevant. (Of course, this number is only one heuristic. A full evaluation has to consider the sentience of each organism, the cause of death, lifespan, etc.)
There's tons of useful info in this piece.
:) Thank-you!
I take it that your "Life span" section refers to adult lifespans?
No, this section refers to total lifespan, except where specifically noted. So 20 days is maturity AND death in 20 days (some species of aphids at very warm temperatures).
Do you have estimates for life expectancy at birth (maybe ignoring egg mortality, assuming eggs aren't sufficiently sentient to warrant concern)?
No, this is hard. Average life expectancy at birth will vary wildly between species of terrestrial insect herbivores because of large variation in both maximum lifespan and survivorship.
Your sections on "Predators" and "Parasitoids" gave some point estimates based on when predation and inoculation by parasitoids often occur. Maybe those are reasonable approximations for life expectancy at birth.
These are lifespan expectations for individuals killed by predators or parasitoids only (and are really only gross generalizations), so they don't represent average lifespans.
On the other hand, isn't survivorship almost always "concave upward", with most deaths occurring quite early? This figure is one random example, showing that most of the insects are dead before the second instar. And because of the concave-upward shape, the average age of death should be pretty young.
In short, no. As we state, the survivorship curves are wildly variable depending on the species, the location and the year. For examples of the variability please see Fig 2 from Cornell & Hawkins 1995 and Fig 1 from Hunter 2000. Different groups have different major causes of mortality, which lead to different curves. For example, endophytic species that are not leaf miners have very low mortality at the youngest ages, and experience the most loss at late juvenile or pupal stages.
I tend to assume that insects in diapause have relatively little subjective experience, such that those periods of time "don't count" very much if we're using lifespan as a measure of how long the animal experiences pleasure and pain. Of course, if the insect is minimally sentient during that time, then maybe deaths occurring during that time aren't that bad.
I would be uncomfortable making this generalization. There is a gradient from simple behavioral inactivity to deep diapause, and the mechanisms of diapause are quite variable even within species groups (e.g., Hand et al. 2016).
Extending this idea, it seems plausible that ectotherms that mature slowly in cool climates have less sentience and less hedonic experience per day than those in warm climates, because biological activity is generally slowed down in cool climates. So maybe the difference in total amount of life experiences is less than one might assume between longer-lived slow-developing insects in high latitudes vs fast-developing insects at low latitudes.
This is almost certainly incorrect. A species that lives in a cool climate does not necessarily have an average experienced daily temperature that is less than a species in a warmer climate, except for really extreme cases (e.g., like comparing yearly average of species in the arctic to those at low elevation in the tropics). The temperatures experienced by insects are determined by their microclimate, which will vary with species and habitat type even on vanishingly small scales (e.g., Pincebourde & Casas 2019. It might be better to attempt such generalizations by species groups, but even that is not going to be easy (e.g., soil insects in closed canopies will experience cooler temperatures on average in the summer than leaf feeders open canopies, but warmer temperatures in the winter).
If we imagine only two species of insect -- one with lifetime fecundity of 2 and one with 4000 -- and if each species has equal numbers of egg-laying mothers, then the ratio of (total offspring)/(total mothers) will still be very high: (2 + 4000)/(1 + 1) = 2001. When we make assessments about the net hedonic balance of an entire ecosystem containing multiple species, it's this average value that seems most relevant. (Of course, this number is only one heuristic. A full evaluation has to consider the sentience of each organism, the cause of death, lifespan, etc.)
Please note that these values are extrema and are not a good representation of the distribution. The median fecundities are reported and are the best representation of the central tendency (overall 138), since the medians are seriously left-shifted from the max. Therefore, the majority of individuals will be born to parents with median fecundity.
References Cornell, H. V., & Hawkins, B. A. (1995). Survival patterns and mortality sources of herbivorous insects: some demographic trends. The American Naturalist, 145(4), 563-593. https://www.journals.uchicago.edu/doi/abs/10.1086/285756
Hand, S. C., Denlinger, D. L., Podrabsky, J. E., & Roy, R. (2016). Mechanisms of animal diapause: recent developments from nematodes, crustaceans, insects, and fish. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 310(11), R1193-R1211. https://www.physiology.org/doi/full/10.1152/ajpregu.00250.2015
Hunter, A. F. (2000). Gregariousness and repellent defences in the survival of phytophagous insects. Oikos, 91(2), 213-224.https://doi.org/10.1034/j.1600-0706.2000.910202.x
Pincebourde, S., & Casas, J. (2019). Narrow safety margin in the phyllosphere during thermal extremes. Proceedings of the National Academy of Sciences, 116(12), 5588-5596. https://www.pnas.org/content/pnas/116/12/5588.full.pdf
Interesting. :) When I said "I tend to assume that insects in diapause have relatively little subjective experience", I had in mind the prototypical case of diapause where metabolism dramatically decreases.
I see that Hand et al. (2016) make the point that diapause doesn't always imply reduced metabolism: "Diapause [...] may or may not involve a substantial depression of metabolism" and "Diapause [...] depending on the species, can also be accompanied by depression of metabolism, essential for conserving energy reserves."
When I was reading about diapause, most of the sources suggested that metabolism was reduced, so I assumed that was the usual case. For example: "During diapause an insect's metabolic rate drops to one tenth or less".
Thanks for the further insights. :)
I wasn't very clear about the phrase "adult lifespan", which I was probably using incorrectly. What I had in mind was "average lifespan only counting individuals who survive to adulthood", which I think is similar if not the same as what you had in mind.
Life expectancy at birth may vary a lot, but I think it'd be interesting to see some example numbers to get a sense of the diversity, similar to how you gave lots of other sample numbers for other metrics. I assume one could compute it from survivorship curves. (This is just a general point for future work that people might do. You've already gathered a huge amount of info here, and I don't mean to request even more. :) )
My comment was partly inspired by this quote from your piece: "Species from cool temperate regions tend to have longer life cycles with about one generation per year (e.g., Danks and Foottit 1989), as do species living in areas that have a dry season. But we note that for many of these species, variable environmental conditions determine how many generations there are per year, and in addition, the overwintering generation will have a longer lifespan than growing season generations." I didn't read the source articles, but I was guessing that when species have longer lifespans due to cold or dry conditions, they presumably have to slow down metabolically during those unfavorable periods. And metabolic slowdown presumably means that activity by the nervous system slows down too.
I tried Googling about that and stumbled on Huestis et al. (2012). The authors expected mosquitoes to reduce metabolic rate during aestivation like happens for insects during winter diapause, but resting metabolic rate was actually higher during the late dry season. "The high ambient temperatures during the Sahelian dry season may prevent or limit a reduction in metabolic rate even if it would be adaptive."
Still, it does seem true that insects experiencing cooler temperatures typically slow down metabolism (with your point taken that one has to consider microclimatic temperature). So I guess my point here reduces to the previous point about how winter-diapausing insects (as well as those experiencing reduced temperatures even not in diapause) plausibly matter less per unit time, in proportion to the extent of slowdown (leaving room for lots of exceptions and diversity depending on the details).
Very interesting about warm-weather diapause and metabolic rate for mosquitoes. I'll agree that during deep cold-weather diapause insects are reducing metabolic rate (goodness, but maybe not when REALLY cold??). A quick lit search turned up seasonally variable brain size and cognitive abilities in shrews (Lázaro et al. 2018)!
No idea how this relates to lived experience tho. Extending this argument, would you also claim that species with slower metabolism have less lived experience than those with faster metabolism (e.g., "less sentience and less hedonic experience per day"), because then comparing between species with different metabolic rates is going to be quite difficult. In fact I think it quite likely that those species with faster metabolic rates have different lived experience rates than species such as humans, e.g., Healy et al. 2013.
Healy, K., McNally, L., Ruxton, G. D., Cooper, N., & Jackson, A. L. (2013). Metabolic rate and body size are linked with perception of temporal information. Animal Behaviour, 86(4), 685-696. https://doi.org/10.1016/j.anbehav.2013.06.018
Lázaro, J., Hertel, M., LaPoint, S., Wikelski, M., Stiehler, M., & Dechmann, D. K. (2018). Cognitive skills of common shrews (Sorex araneus) vary with seasonal changes in skull size and brain mass. Journal of Experimental Biology, 221(2), jeb166595. https://jeb.biologists.org/content/jexbio/221/2/jeb166595.full.pdf
That shrew thing is fascinating!
Yeah, as an initial hypothesis I would guess that faster brain metabolism often means that more total information processing is occurring, although this rule isn't perfect because the amount of information processing per unit of energy used can vary. Also, the sentience or "amount of experience" of a brain needn't be strictly proportional to information processing.
In 2016 I wrote some amateur speculations on this idea, citing the Healy et al. (2013) paper.
Very interesting! Is the dung beetle fecundity two per female? How can the population ever grow?
Gilbert and Manica (2010) list Kheper nigroaeneus as the dung beetle with the lowest median lifetime fecundity, but do not in their manuscript or supplemental materials attach a reference to the individual data points in their meta-analysis. I suspect the source for this data point is Edwards & Aschenborn (1989). In the supplemental data Eurysternus balachowskyi is also listed with lifetime fecundity of 2. My best guess at a reference here is Halffter et al (1980).
Edwards & Aschenborn (1989) report that female Kheper nigroaeneus lay one egg per year, but will lay another if the larvae is unsuccessful early on. The authors further note that that "To ensure population replacement, females must live at least into a second year. We have indirect evidence (laboratory studies; tibial wear measurements) that some adults survive into a third year." In other words, occasionally a female will produce 3 offspring.
On the other hand, there are a fair number of dung beetles listed as endangered (at least in 1st world countries, e.g. references in Buse et al. 2015, Carpaneto et al. 2007) due to habitat loss, fragmentation and reduction or extinction of associated mammal species.
Interesting post. I was wondering if you could clarify what you'd include in the terrestrial insect herbivore grouping a bit more precisely. It's not defined in your post and might be a bit ambiguous to non-biologists, for instance:
-Are freshwater aquatic insects (or those with aquatic juvenile forms) included?
-Are species with winged adult forms included?
-Are nectavores included?
-Are insects that switch between being carnivorous and herbivorous between juvenile and adult stages included (I'm not sure there are many examples of this, maybe some ants)?
Also, you mention parental care in the context of fecundity but not juvenile mortality. I assume that parental care would drastically reduce juvenile mortality, is that correct?
Sure, so included/excluded unless there is a specific statement otherwise WRT to a particular study: 1. strictly terrestrial species, no aquatic juveniles, 2. lots of species with winged adults, 3. nectavores in included. 4. As far as I know no species which switch between carnivorous and herbivorous.... I'm struggling to think of examples, but my guess would be that these belong to eusocial species, which are not really dealt with here.
Parental care in a particular group does reduce mortality in general compared to the average rates in that group. I can't speak to comparisons across groups tho. So for example, don't know, but don't think that, in general, parental care in exophytics decreases mortality below that of endophytics.
Thanks! Ok terrestrial is pretty clear but herbivorous still throws up a lot of edge cases (although I do appreciate the focus of this article is on classic herbivores).
For instance, I thought of parasitic wasps/flies and social wasp with regards to the last point in my previous post. These often have nectivorous adults that predate other insects as food for their larvae.
Some other questionable herbivores are:
-Opportunistic carrion foraging by tropical bees.
-Consumers of animal waste products (I saw you included dung beetles as herbivores). There are also moths that drink the tears of sleeping birds, and dust mites eat shed skin cells.
-Insects that steal the stored plant products of other animals. Many bee species are known to raid honey from other bee colonies, although while some tropical species are known to do this frequently I'm not sure any do so exclusively. Bees on both sides are usually killed during the raids.
-Carnivorism that is primarily for aggressive reasons rather than to fulfill a dietary need. For instance, queens and worker in bee colonies will eat worker laid eggs they find.
We've tried to keep it clean with respect to data on herbivores, and in most cases have noted where the reference deals with other data as well.
For example, we do not include dung beetles in our definition, and you'll see above where we note the fecundity of these reported by Gilbert and Manica (2010a), but then ferret out the herbivore-specific extremes from their supplemental data (their medians and stats DO include non-herbivores tho). (Note: my bad, I just spotted that sentence didn't make the final cut... I've added it back into the post for greater clarity).
Dust mites would not be included here since we deal only with Insecta, although certainly some of the lifespan papers that are referenced in a more general way (e.g., Convey 1997, Danks 2006) also have data on mite species.
Not so sure that some of the edge cases really constitute a deviation from herbivory unless there is a significant portion of the diet involved (e.g., cows eat a lot of insects). However, Hawkins et al 1997 and Cornell & Hawkins 1995 claim all the species they examine are terrestrial herbivores as classified by larval stage. I can't speak to these specific examples for bees, other than to note that the percentage of Hymenoptera in the review papers is small (mortality:6%; fecundity: ~12%).
On the other hand, I've started arguing in the scientific lit that this type of trophic classification is always context-dependent anyway.
Really interesting article!
I think that 17 and 19 should be exponents :)
Indeed! If anyone has a way to do add superscript in this version of markdown, I would be grateful to know!!
This might be squints 4 years too late, but one can always use unicode superscript characters, in this case "10¹⁷-10¹⁹".
Or one could use LATEX (slightly more involved), writing
10^{17}-10^{19}, which results in 1017−1019. Perhaps a bit out of place in this text :-)Where would starvation fall under in the juvenile mortality pie chart? Or is that something r-selected species don't typically go through.
It would be under competition in the pie chart. It is a lowish percent of total mortality.
Do you think it would be different for detritivores compared with herbivores? Given that many plants aren't significantly consumed by animals, it seems there is often food in existence for herbivores to eat. In contrast, almost all decomposing organic matter will eventually be eaten by someone or other, so that food source could run out (or, if food doesn't run out, then maybe water does during dry periods). That said, maybe insect decomposers are still limited in number by factors like predators and parasitoids, and it's the other decomposers (bacteria, fungi, etc) who mainly face the resource limits.
I think its going to differ between species groups and habitats within the classification of detritivore. The fact that plants are not significantly consumed by terrestrial herbivore suggests that there is lots of dead and decaying material about for terrestrial detritivores. Moreover, the plants keep producing more, so its not like it runs out (at least not unless there is a major perturbation). Of course there are issues like C:N ratios but most (all?) detritivores consume microbes and fungi as part of their diet as well. The situation may be different for species that focus on other resources (dung beetles), and certainly in aquatic systems there is often no significant standing stock of primary producers, so there could be limitations there (e.g., studies on stream leaf shredders often point this way), although in open pelagic systems the "standing stock" is really secondary production, and that is what detritivores would focus on.