Last year, I wrote about the promise of gene drives to wipe out mosquito species and end malaria.
In the time since my previous writing, gene drives have still not been used in the wild, and over 600,000 people have died of malaria. Although there are promising new developments such as malaria vaccines, there have also been some pretty bad setbacks (such as mosquitoes and parasites developing resistance to commonly used chemicals), and malaria deaths have increased slightly from a few years ago. Recent news coverage[1] has highlighted that the fight against malaria has stalled, and even reversed in some areas. Clearly, scientists and public health workers are trying hard with the tools they have, but this effort is not enough.
Gene drives have the potential to end malaria. However, this potential will remain unrealized unless they are deployed – and every day we wait, more than 1,600 people (mostly African children) die. But who should deploy them?
I’m well aware of the Unilateralist’s Curse, and before publishing this post, I asked, why haven’t existing groups already released gene drive mosquitoes? Academic labs, and the nonprofit organization Target Malaria, have been working on gene drives for several years already, but they have been taking a cautious, incremental approach.[2] There are several good reasons for this:
- Unilateral release of a gene drive is likely to cause public backlash, because genetic engineering is widely perceived as scary. This is especially true if the organization releasing the gene drive does not have support from the local government.
- The first version of any gene drive released into the wild is unlikely to cause complete eradication because mosquitoes may become resistant (either through natural or nuclease-induced mutations).[3] Therefore, updated gene drive versions will likely be required to overcome resistance. The number of potential gene drive target sites in the mosquito genome is big enough that I don’t think this will stop eradication, unless gene drives are banned after the first release due to public backlash.
- As a corollary, a temporary drop in malaria due to a partially effective gene drive, followed by resurgence due to resistance, could lead to even more deaths because humans would lose protective antibodies against malaria over time.
- Gene drives also have the opportunity to eradicate other pathogens such as dengue fever (spread by Aedes mosquitoes) and schistosomiasis, and if they are banned, this opportunity will be wasted.
Basically, this means that any organization releasing gene drives needs to ensure they are not banned. This would be difficult for a private organization, as influencing public opinion on GMOs is hard. However, if a government did this, it could simply choose not to ban gene drives (though it would need to remain firm against possible international pressure).
With that out of the way, I want to explore the technical details of gene drive construction, and describe what it would take for a moderately-well-equipped biology lab to build one targeting Anopheles gambiae. I emphasize that this is still a draft plan, and although I am skilled in molecular biology, I am not a mosquito specialist. But given the immense amount of death and suffering caused by malaria, I hope that the information I provide can help bring an end to this menace, by highlighting that political concerns, not technical challenges, are the main barriers to gene drive deployment.
How to make a gene drive
First, I want to thank Andrea Crisanti and his lab, not only for developing Anopheles-targeting gene drives, but also for making many of the technical details (including DNA sequences and mosquito editing methods) publicly available. This plan closely follows their strategy for making a gene drive.
There are three main steps: first, constructing the gene drive DNA; second, integrating it into mosquitoes; and third, releasing the mosquitoes into the wild.
The DNA
In 2020, the Crisanti lab reported a highly effective doublesex X-shredder gene drive, and helpfully, they published the DNA sequence for it. Note that it may be possible to make improvements on this gene drive,[4] but the overall process will be very similar regardless of which gene drive version is used.
The basic structure of the gene drive is shown in Figure 1 of the paper describing it:
The key functional parts are the SpCas9 nuclease driven by the germline-specific vas/zpg promoter,[5] the I-PpoI nuclease[6]driven by the male germline-specific Beta2 promoter,[7] and a guide RNA, driven by the constitutive U6 promoter. In the paper, the authors tested several versions of the drive, and found that one with a gRNA targeting the doublesex gene performed the best.[8] The drive version in the paper also contains fluorescent proteins to allow easy identification of drive-positive mosquitoes. However, these are functionally unnecessary, and a smaller gene drive payload will be more efficient, so it would be better to omit these.[9]
To integrate the gene drive DNA into mosquitoes, the Crisanti lab took a two-step approach. First, they performed a CRISPR knock-in of a small “landing pad” sequence containing attP recognition sites for φC31 integrase, as well as GFP under control of an eye-specific promoter[10], into the doublesex gene. Next, they performed recombinase-mediated cassette exchange using a φC31 integrase plasmid and the gene drive plasmid, which contains attB sites that the φC31 enzyme recombines with the attP sites that were added in the first step. To follow this approach, we[11] will need four plasmids:
- The gene drive plasmid or a smaller version without fluorescence proteins
- The φC31 integrase plasmid (a Drosophila version can be obtained from Addgene, which will likely work in mosquitoes too)
- The landing pad plasmid.
- A Cas9/sgRNA plasmid for knocking in the landing pad. This can be obtained by using Golden Gate (SapI) cloning to add the sgRNA sequence into an existing plasmid from Addgene.[12]
Because we know the sequences for all of these, we can order them from a commercial DNA synthesis provider such as Genscript, IDT, or Twist (all of which I’ve used for my own research). I got a quote from Genscript for $5600 to synthesize the smaller version of the gene drive plasmid, and $6500 for the larger version with the fluorescent proteins. If money is very tight, it would be cheaper to order the plasmid in smaller fragments and combine them using Gibson assembly. In this case, the DNA would cost approximately $900 for the small version and $1100 for the large version, although labor costs would be considerably higher.[13] In any case, assembling these plasmids, growing them in bacteria, and preparing DNA for injection will require only bachelor’s degree-level molecular biology work, one to two months of time, and at most $25,000 in DNA, reagents, and lab equipment.[14] Things will start getting more expensive in the next step.
The Mosquitoes
This project will require a mosquito breeding facility. If we have a permit, we can obtain Anopheles mosquitoes from a research repository, but I’m going to assume this will take place in an African country where we can catch mosquitoes from the wild as a backup option.[15]
For some weird reason,[16] the International Atomic Energy Agency publishes a 44-page guide on Anopheles mosquito husbandry. It covers quite a lot of details, including how to collect mosquitoes from the wild, how to build breeding cages, how to set up feeding stations (with sugar water, and cow blood for females after mating), and how to prevent common problems such as ant infestations. It also includes a list of references for more information on particular steps.
For even more detail, we can look at the comprehensive 408-page “Methods in Anopheles Research” published by the American Type Culture Collection and the NIH (chapters 1–4 are relevant for mosquito breeding, and it also covers mosquito microinjection). This also has a catalog of entomology suppliers, which will be very useful. The basic three-week breeding cycle is as follows:
Friday: Blood-feed adult females. The mosquitoes should be a minimum of two days post-emergence for the best results.
Monday: Insert the egging dish into the cage.
Tuesday: Remove the egg dish from the cage. Bleach the eggs and store them in a humid sealed cup overnight.
Wednesday: Rinse eggs into pans for hatching and feed.
Friday: Split the larvae into pans based on the number you will need but keeping in mind proper densities. Add yeast to a final concentration of 0.02% w/v and a very small amount of the larval diet you will use.
Sunday: Feed the larvae a volume of ground diet based on their size and density. If there are too many larvae in the pan, thin or split into more trays to ensure no crowding occurs.
Monday through Wednesday: Continue splitting/thinning and feeding the pans daily as needed. It is best if the density at this point is the same as the final density; crowding slows development.
Wednesday through Friday: Pupae should be collected daily and transferred to a cup with clean water and placed into a new cage with a sugar source.
Friday of the following week: Bloodfeed the adults to initiate the cycle again.
I’m not going to do a detailed calculation of how much it would cost to build a mosquito breeding facility, but I’d roughly estimate somewhere around $100,000 in equipment (not counting the building itself), and two employees to run it. The time required to get it up and running would probably be around 6 months of intense work, which could be done in parallel with the plasmid preparations.
Editing and Release
Although setting up a breeding facility will be operationally difficult, the most technically challenging part of this whole project will be doing the actual mosquito editing. Fortunately, the Crisanti group published a helpful guide on how to inject DNA into mosquito eggs to perform editing. This is all explained pretty well in the guide (they even include catalog numbers of key equipment to buy), and I don’t have much to add here. The following equipment will be required (prices are from suppliers):
- Inverted fluorescence microscope (roughly $10,000 including light filters)
- Microinjector (roughly $10,000)
- Micromanipulator (roughly $2,500)
- Microinjection pipettes (roughly $500)[17]
- as well as miscellaneous inexpensive equipment[18] (probably another $5,000)
I’d estimate the total equipment cost to be roughly $28,000. After obtaining the equipment, the basic steps are to:
- Collect freshly laid mosquito eggs
- Line up the eggs on a microscope slide
- Inject the DNA using a microinjector[19]
- Grow the mosquitoes and screen for successful transformants
It will probably take a few months of practice to get right, and it would be a good idea to practice using a simple plasmid to make the embryos express GFP, before moving on to more complicated editing. We will need to do two rounds of editing: one to introduce the attP “landing pad” and another to swap out the “landing pad” for the gene drive. Then, we will need to breed gene drive mosquitoes. This could be a bit annoying due to the tendency of the gene drive to crash populations, so we will need a good supply of females to expand the numbers. Because of the PpoI nuclease X-shredder design, we will know our gene drive is working when all the larvae are males. Releasing a few thousand males would be sufficient to introduce the gene drive allele into the local population. Mosquitoes lay about 100 eggs each and the breeding cycle is 3 weeks long, so starting from a single edited mosquito it would take only a few months to breed the required numbers. I’d estimate another 6 months for this phase of the project if everything goes perfectly – which it probably won’t, so maybe 12 months is more realistic.
Post-Release Monitoring
After releasing the gene drive, we will need to check for resistance in order to develop updated drives to defeat the resistance. This will involve capturing mosquitoes from the wild (assuming there are any left), extracting their DNA, and sequencing it. PCR amplification of the target site, followed by Sanger sequencing, should be sufficient to test for resistant alleles at the target site at a cost of about $5 per sample. For larger numbers of samples, we could do the PCR in a barcoded format, pool samples, and do next-generation sequencing. This could scale very well (approximately 1 cent per sample, assuming batches of 250,000) but would require a larger upfront cost. Still, at this point the bigger cost would be collecting mosquitoes.
It’s also possible that resistance could arise due to mutations elsewhere in the genome, so if we observe that mosquito populations are rebounding, we should perform whole genome sequencing, at a cost of about $500 per sample.
Post-release monitoring may actually be the most expensive part of the project, because if the gene drive works, it will spread internationally, requiring collection of mosquitoes from a large area. Monitoring should continue until eradication is achieved, which may take a few years depending on mosquito migration rates.[20]
Public Relations
Since people will eventually find out about this project (even if it’s initially secret, they’ll notice when mosquitoes start disappearing), it’s best to take a proactive stance on public relations to mitigate backlash. This will be equally, if not more, important in comparison with the rest of the project, since we really want to avoid getting gene drives banned. Non-gene-drive transgenic mosquito technology has been accepted in several countries,[21] and an important part of this was convincing people of the benefits. We should emphasize the project’s potential to end the vast amount of death and suffering caused by malaria. As a top scientist of Target Malaria recently expressed in a news article:
We may not know what may happen but we know what is happening today: 600,000 people dying of malaria, and we need to fix it,” said Dr. Diabaté, the principal investigator in Burkina Faso for Target Malaria, a project backed by the Bill & Melinda Gates Foundation. “We can’t say we are afraid of the future so we will accept 600,000 people dying. We make good progress as a society when we invest in our dreams, rather than our fear.”
A call for political action
Overall, I think this gene drive could be built and released for under a million dollars (considering equipment and salaries), although a budget of a few million would allow the project to move faster by hiring more people. Of course, the reason this is so cheap is that the R&D costs have already been paid by the Crisanti lab and others. I want to emphasize that releasing a gene drive, without having the R&D capacity to make a followup drive in the likely case that the first drive is insufficient, will be doing more harm than good. So, realistically this would be a multi-million dollar project.
But I also want to emphasize that this is well within the capabilities of most African governments! Just to pick a random sub-Saharan country, the national revenue of Ghana is about 65 billion Ghana Cedi, or $5.5 billion USD at October 2023 exchange rates.[22] Funded at $5 million per year, this would be about 1/1000 of total government revenue – and I’m sure the Ghanaian government could get some foreign support if it asked. For a larger country like Nigeria, it would be a trivial expense.
Thus, the barriers holding back gene drive deployment are political, not technical. A recent news article covering gene drives observed that:
[a project in São Tomé and Príncipe led by the University of California[23]] needs government approval to move forward with the genetic portion of the intervention and São Tomé and Príncipe, like many other African countries, does not yet have a legal framework for the use of genetically modified organisms. Legislation to establish one has stalled in the National Assembly. Without a body assessing the risks and safety of using a tool like these mosquitoes, the California team has no one to submit its project proposal to and is effectively stalled.
So, assuming you’re not an African government official,[24] what can you do to help? I’m not a politician or PR expert, but here are some ideas:
- Coordinate with existing gene drive programs and fund PR campaigns to support their efforts.
Send an email to a Nigerian prince about your unique investment opportunityIdentify which African government officials have the power to approve gene drives in their countries, and encourage them to support this.- Advocate for reasonable international regulations on gene drives for disease vector species.[25]
- Advocate for the development and testing of more limited transgenic mosquito technology (non-gene-drive mosquitoes, or gene drives which self-inactivate after a set number of generations) as a way of gaining acceptance for a full-scale gene drive by showing that these limited versions are safe and effective.
- Spread the message that ending malaria is possible if we try.
If you have a typical reading speed,[26] in the time it’s taken you to read this far, thirteen more people have died of malaria. Gene drives can end this ongoing tragedy, but only if we decide to use them.
I thank Devon Stork for his helpful feedback on a draft of this article.
- ^
See: https://www.nytimes.com/2023/09/29/health/mosquitoes-malaria-disease-climate-change.html and https://www.nytimes.com/2023/09/29/health/mosquitoes-stephensi-malaria-africa.html
- ^
The last news from Target Malaria was in 2022, when they reported results of releasing non-gene-drive sterile male mosquitoes in Burkina Faso. As far as I know they have no active plans to release gene drives, not even limited “split drive” or “daisy drive” versions.
- ^
Even target sites with a low expected fitness of resistant alleles (such as doublesex) still have this possibility, and the mosquito population is very large.
- ^
Interestingly, a simulation paper found that a more basic version of the gene drive without the PpoI X-shredder may be better at spreading through heterogeneous populations. Basically, if the fitness reduction is too high, the drive allele dies out too quickly before spreading. But, the X-shredder drive has a lower chance of resistant alleles forming. As a reminder, the first gene drive release is unlikely to be completely successful, so it’s important to not have gene drives get banned!
- ^
Surprisingly, they seem to be using a version of Cas9 codon-optimized for human expression instead of insect expression.
- ^
This nuclease shreds the X chromosome, making all sperm male.
- ^
They found that a slightly modified promoter with lower activity performed better.
- ^
doublesex is a highly conserved gene involved in sex determination. When the intron 4–exon 5 splice site is disrupted, females cannot develop properly but males are fine. Because the sequence is so conserved, doublesex drives have a chance of spreading into other Anopheles species besides A. gambiae if hybridization takes place. This is probably a good thing, as those other Anopheles species also spread malaria.
- ^
Furthermore, one hypothetical resistance mechanism is mosquitoes evolving to not mate with fluorescent mosquitoes.
To make the version without fluorescent proteins, I downloaded their DNA file and opened it in Benchling. I highlighted the gene drive sequence in the screenshot below.
The rest of the sequence is for allowing the plasmid to be propagated in bacteria. At 14,465 base pairs, it’s a relatively big plasmid. I also designed a version of the plasmid with non-functional elements removed (including the fluorescent proteins), making the gene drive sequence about 20% smaller (the bacterial sequence is unchanged).
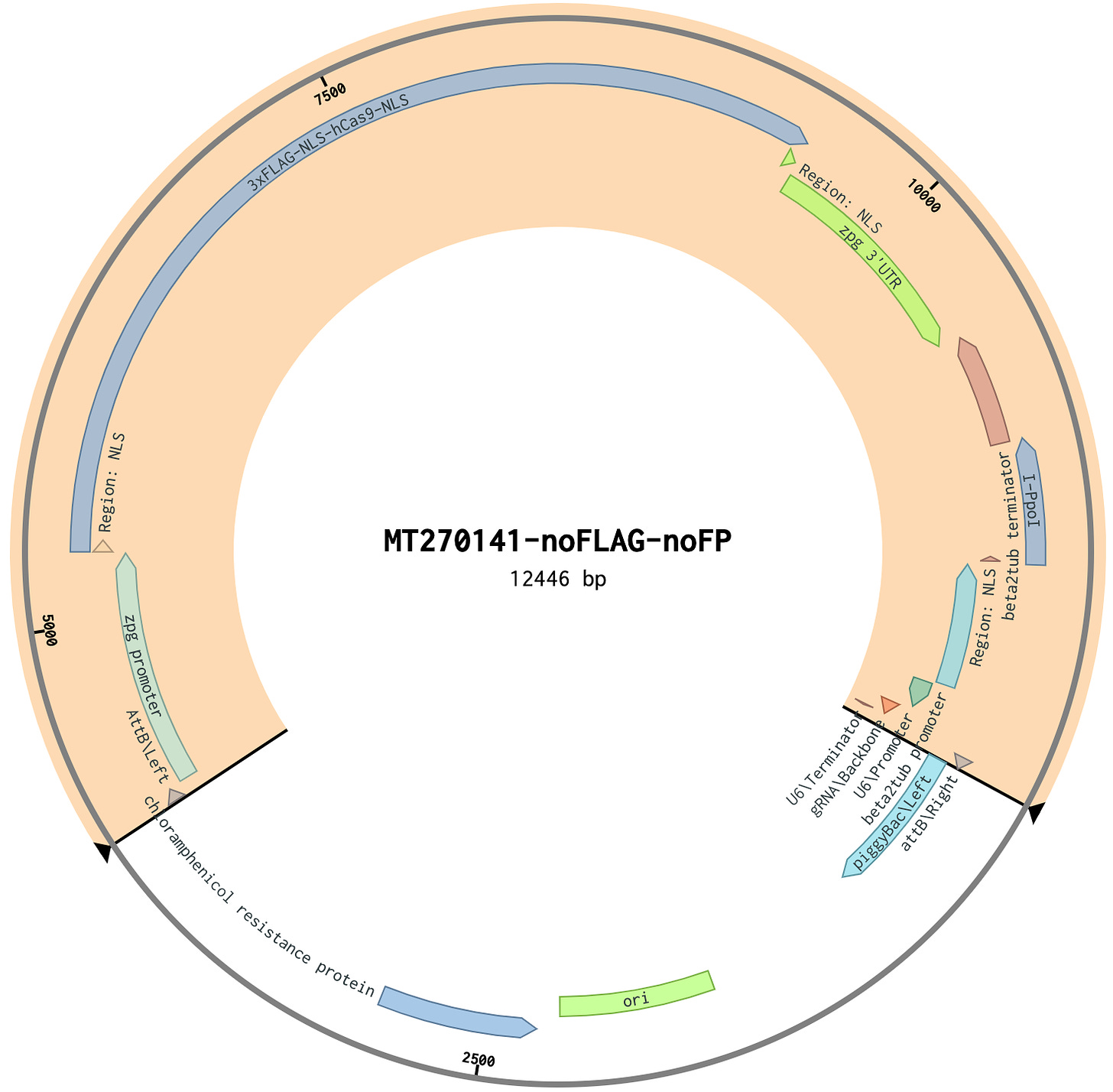
- ^
This is very helpful for identifying mosquitoes where the integration was successful. Also, at the second stage where the gene drive sequence is integrated into the doublesex site, loss of GFP indicates success.
- ^
I’m going to be using “we” here to mean the people building the gene drive, and this does not indicate that I will be personally involved.
- ^
Or possibly through modifying the gene drive plasmid, which already expresses Cas9 and an sgRNA targeting doublesex, to have the constitutive promoter from the φC31 plasmid driving Cas9 instead of the germline-specific zpg promoter.
- ^
If I did this personally, it would probably take me about a month of lab work. If I ordered the plasmids instead, it would take 2 days of hands-on work.
- ^
Centrifuges, incubators, etc. Note that it will be important to have 24-hour electricity which many African countries do not have, so expenses for a generator will also likely need to be added.
- ^
Doing this in an area where mosquitoes are endemic means that if any mosquitoes escape, they might spread the gene drive. This has generally been considered a bad thing, but if we’re planning to release them anyway, it’s not terrible – except for the PR problems it would cause.
- ^
It’s because radiation is used to sterilize mosquitoes for population suppression.
- ^
Alternatively we could buy a micropipette puller for about $5,000 and have a functionally unlimited supply.
- ^
A dissection microscope, pipettes, Petri dishes, filter paper, strainers, etc.
- ^
I learned how to do this on frog and mouse eggs at Frontiers in Reproduction. It took me a few days to learn, but I was helped by an experienced teacher.
- ^
Migration rates, not breeding rates, will be the limiting factor here. See https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4306333/ and https://pubmed.ncbi.nlm.nih.gov/9439109/ for differing estimates.
- ^
To be specific, Oxitec’s sterile Aedes mosquitoes.
- ^
Although, the budget document indicates Ghana is at a high risk of default on its foreign loans. Still, the expenditure would be small compared to other government programs, and would have a high rate of return.
- ^
This project is funded by Open Philanthropy and intends to use a gene drive not to eradicate mosquitoes, but to make them express anti-malaria antibodies and not transmit malaria. However, a gene drive expert privately expressed various concerns to me about this project, such as that it will likely spread outside its intended area (the islands of São Tomé and Príncipe) to mainland Africa, and cause backlash in mainland countries leading to gene drives being banned. Furthermore, it may cause resistance in mosquitoes, or the evolution of malaria strains that are resistant to the anti-malaria antibodies. I hope the project can address these concerns before deploying their gene drive.
- ^
And if you are an African government official, I’d love to talk with you about this!
- ^
Although this is less likely to work than getting one country to allow gene drive release, it is still worth trying.
- ^
(2848 words, not counting footnotes) / (250 words/minute) * (600,000 deaths in last year) / (525600 minutes in last year) = 13 deaths

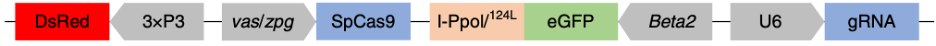

Executive summary: The author argues political hurdles rather than technical challenges are preventing deployment of CRISPR gene drives to eradicate malaria-carrying mosquitoes.
Key points:
This comment was auto-generated by the EA Forum Team. Feel free to point out issues with this summary by replying to the comment, and contact us if you have feedback.